From AFFF to F3 : History – Part 1
Modern chemistry has created many hundreds of thousands of chemical compounds, which we encounter as part of our daily lives. Indeed, it would be very hard to spend a day without being in contact with a class of molecules – perfluoroalkyl substances or PFAS – which have been commercially exploited since the end of WWII over the last 75 years.
Even if we could qualify chemistry as a miracle of science, it is worth knowing that chemistry has been approached by the Egyptian 3000 years BC, and was later on studied by the Ancient Greeks which described the combination of the 5 elements: earth, air, fire, water and ether. This theory was largely accepted for more than 1000 years.
The basis of the modern chemistry, as we now understand it, was established over the last three hundred years. The nature of the atom, the identification of atomic compounds, the first modern synthesis was achieved.
In 1906, Frédéric Henri Moissan (1852-1907), a French chemist working in Paris at the École Supérieure de Pharmacie, isolated for the first-time elemental fluorine gas, F2, a discovery for which he was awarded the Nobel Prize in Chemistry in 1906. Hydrogen fluoride obtained from fluorspar had been identified by the renowned Swedish chemist Karl Wilhelm Scheele some years earlier.
The post war years after WWI In the mid 1930s, were the heyday the German chemical industry, especially as regards new synthetic dyes. Fluorine chemistry started to have a commercial role – a red dye Naphthol AS, used as the official red colour the Nazi flag, and Indanthrene Blue used as a component of ‘Flieger Grau’ or Pilot Grey, the blue-grey colour of Luftwaffe uniforms, both contained a fluorinated methyl group, CF3, which helped prevent fading. Carothers working for DuPont produced the first industrial wholly synthetic fabric, the polymer Nylon. From this point chemists have never stopped to inventing new compounds!
When we think about chemicals, it is important to realise that many, if not most of the synthetic compounds now commercially available are produced by the petrochemical industry. Since the invention of the internal combustion engine, petrol (gasoline) and petroleum products have been become increasingly important around in for many human activities but are associated with a high fire risk.
The nature of this risk highlighted the necessity of addressing these often-catastrophic fires. In the early 40’s, protein foam made from ‘horn and hoof’, a slaughterhouse waste, was developed to control Class B hydrocarbon fires, e.g., those involving oil, gasoline, aircraft fuel and solvents.
In 1949, the 3M Company Minnesota industrialized the Simons electrochemical fluorination (ECF) process for making perfluoro-compounds (PFC) such as perfluorinated amines, carboxylic and sulphonic acids in which the hydrogens of the alkyl carbon chain had been totally replace by fluorine. Joseph Simons had discovered the ECF process whilst working at Pennsylvania State College in the 1930s but was unable to publish his work until after WWII because fluorine chemistry was essential for uranium purification as part of the Manhattan Project.
In 1953 the structure of Scotchgard was accidentally discovered by Patsy Sherman and Sam Smith working for the 3M Company whilst working on a rubber for jet fuel lines. Three years later in 1956, the 3M Company launched Scotchguard on the market. This fabric, textile and leather treatment is based on a PFOS-derivative containing N-ethyl-PFOSA and gives water, oil, and other liquids and stain-protection to the treated fibre.
Interestingly, N-ethyl-PFOSA known as Sulfluramid, was originally developed to kill ants, cockroaches and termites, and is still used to this day as the insecticide sulfluramid against leaf-cutting ants in Brazil. The lithium salt of PFOS was developed to kill wasps and hornets but is highly toxic to honey bees.
PFOS, perfluoro-octane sulphonic acid, and its derivatives subsequently become crucial for the development of Class B aqueous film-forming foams (AFFFs) effective against liquid hydrocarbon and solvent fires.
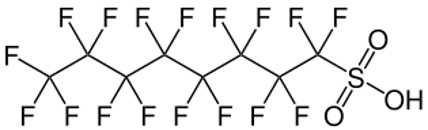
Figure 1. The structure of PFOS, perfluoro-octane sulphonic acid.
During the 1960s the US Department of the Navy Naval Research Laboratory in collaboration with the 3M Company began developing PFOS-based firefighting foams. A patent for AFFF firefighting foam was awarded in June 1996 for extinguishing liquid hydrocarbon fires.
In the late 60’s, a series of major fuel fires happen on board US Navy ships causing extensive loss of life and damage:
(i) 1966: USS Oriskany – fire kills 44 sailors.
(ii) 1968: USS Forrestal – whilst on active service in the Gulf of Tonkin during the Vietnam War, malfunction and accidental firing of a fighter Zuni rocket on the flight deck of this super-carrier led to a catastrophic aviation fuel fire claiming the lives of 134 crew, injuring many more, destroying nearly 50 aircraft, doing $72 million worth of damage, and leaving the vessel unfit for active service.

(iii) 1969: USS Enterprise – a shipboard fire kills 28 sailors.
These major fires prompt the US Department of the Navy to mandate the use of the recently developed AFFF firefighting foam, which the 3M Company was manufacturing for the US military.
Perfluorocompounds had been successfully used to create AFFF, and 3M’s PFOS-based LightWater® and alcohol-resistant ATC® brands became the staple for liquid hydrocarbon fuel fires from the 1970s until May 2000 when the Company announced that it was phasing out PFOS-based chemistry on environmental grounds. AFFF had indeed conquered the world of firefighting and was seen for decades as the ultimate answer to extinguishing large hydrocarbon (oil and gasoline) fires both for military and civilian use especially by the aviation and petrochemical industries.
In the 70’s, an alternative technology was developed based on the telomerization process. This technology provided an alternative to the ECF process and introduced a new class perfluorochemicals on the market. Whereas the ECF process produced mainly PFOS contaminated with odd and even numbered homologues of PFOS such as PFHxS (~5-8 % w/w), perfluorohexane sulphonic acid, as well as branched chain isomers, telomerisation produced only even number linear alkyl carbon chains. The characteristic of fluorotelomer derivates is a perfluoroalkyl moiety linked by a dimethylene group -CH2-CH2- to a functional group which could be negatively (anionic), positively (cationic) or both negatively and positively charged (amphoteric).
Most modern fluorotelomer-based AFFF firefighting foams, known as ‘pure C6 foams’, are based on derivatives of 6:2 fluororelomer sulphonic acid (6:2FTS), or a thioether analogue, containing a C6 perfluoroakyl chain linked through -(CH2)2- to a charged functional group. 6:2FTS contains a C8 chain and its structure is shown below. Its similarity to PFOS is clear but the CH2 groups cause it to behave very differently in terms of its PBT profile. All perfluoroalkyl moieties or their breakdown products are extremely environmentally persistent (vP), but with differing bioaccumulation or toxic potential.
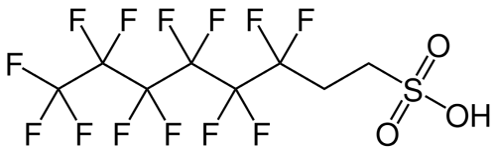
Figure 2. Structure of 6:2FTS
Early fluorotelomer foams, however, contained both 6:2FTS and often substantial amounts of 8:2FTS derivatives. This was a problem as the 8:2FTS could be degraded to a stable end product perfluoroctanoic acid or PFOA which had substantial toxicity. This problem has now been essentially resolved as a result of the industry PFOA Stewardship Program 2010-2015, with residual PFOA or its precursors reduced to less than 25 parts perbillion (ppb).
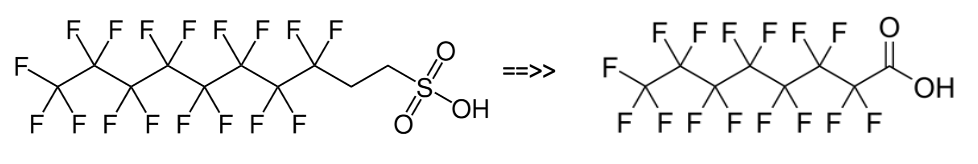
Figure 3. 8:2FTS breakdown to PFOA
From the 1970s to the late 1990s, many manufacturers of firefighting foam appeared in the market, developing and offering a wide range of different foams for end-users. These included Class B AFFF, AFFF-AR (alcohol resistant), film-forming-protein (FFFP) and fluoro-protein (FP) foams. Class A foams specifically intended for solid carbonaceous fires such as structural building or wildland (bush) fires were also developed in this period.
On 16 May 2000 the 3M Company abruptly announced the phasing-out of its activity in PFOS-based chemistry for producing fluorochemicals, affecting not only firefighting foams but also a wide range of domestic and commercial products. This announcement was justified on company responsibility for environment, as it was confirmed that the C8 perfluoroalkyl substances (PFAS) made using ECF technology posed a threat to the environment, with pollution that had spread worldwide affecting a wide range of environmental compartments as well as biota including man.
Over the next 2-3 years, the company had stopped all activities involving PFOS-based chemistry, with a total withdrawal from the firefighting foam market, replacing it with mitigated success by a shorter chain PFBS-based (perfluorobutyl sulphonate) chemistry. However, some production of PFOS and PFHxS derivatives using the ECF process did continue in both China and India.
With 3M’s phase-out of PFOS-chemistry and withdrawal from the firefighting foam market, other major manufacturers of PFAS fluorochemicals and firefighting foam stressed that they considered fluorotelomer chemistry was ’safe’ and indeed environmentally friendly as it had nothing to do with ECF chemistry and products could not contain either PFOS or PFOA. The firefighting foam market transitioned to AFFF products based on fluorotelomers over the next few years 2000-2010.
From 2002 onwards a lively and sometimes acrimonious debate took place between manufacturers of PFAS and AFFF – under the auspices of a trade association, the Fire Fighting Foam Coalition (FFFC), funded mainly by the fluorochemical industry – and independent manufacturers especially of nascent fluorine-free foams (F3), regulators and scientific experts from academia. This discussion gave rise to a series of international seminars, conferences as well as hundreds of publications in the peer-reviewed literature about the environmental consequences of substituting fluorotelomers for PFOS-based products. At this time the main international forum for discussing developments in firefighting foam technology turned out to be the Reebok series of foam conferences, held in Manchester and Bolton in the UK, 2002, 2004, 2007, 2009 and 2013.
Starting as early as 2002 a number of the smaller independent foam manufacturers started to offer first generation experimental Class B liquid hydrocarbon fluorine-free foams (F3) as more environmentally sustainable alternatives to PFAS -containing foams. During the following 10 years the debate raged on driven by published scientific studies which concluded that telomer chemistry posed a threat to the environment. Early telomer formulation were mixtures of 6:2 and 8:2 derivatives. The 8:2 material was shown to be a potential precursor for the generation of environmentally extremely persistent PFOA (perfluorooctanoic acid or C8) through breakdown, subsequently associated with long-term health effects. At the time this was vigorously contested by representatives advocating the fluorochemical industry attending Reebok conferences.
However, as a result of pressure from the US EPA many large feedstock manufacturers adopted the PFOA Stewardship Program 2010-2015 aimed at reducing the use of PFOA or its precursors. Improvements in purifying the fluorotelomer derivatives resulted in a reduction of PFOA related material to less than 25 ppb providing so-called ‘pure C6’ fluorotelomer derivatives. The Stewardship Program led to a change in foam formulations formerly containing C6/C8 fluorotelomers to a so-called ‘drop in’ replacement containing predominantly C6 fluorotelomer.
Unfortunately, this change was not as simple as it was supposed to be and foam manufacturers had to reformulate and increase the total fluorochemical content to achieve a similar performance compared with previous C6/C8 formulations. Unfortunately, end-users were not even made aware of this change!
Around the same time, scientific studies accumulated evidence that even hyper-pure C6 was NOT a suitable alternative, but a ‘regrettable substitution’ and the debate went to another level. 2015 marked a sea-change in the PFAS debate. The toxicity of PFOA was no longer denied by the fluorochemical industry or regulators and the issue was brought to the attention of the public by scientific journalists.
A series of public statements signed by scientists worldwide – the Helsingǿr Statement 2014, the Madrid Statement 2014 and the Zürich Statement 2018 – raised concerns about the continuing use of PFAS and as major planetary pollutants and their long-term impact on the environment. A major publication in 2020 raised the issue that all PFAS should be treated as a chemical class because of their common environmental problems rather than individual chemicals.
The United Nations Stockholm Convention and their Persistent Organic Pollutants Review Committee (POPRC) has added PFOS, PFHxS and PFOA to the appropriate Annexes banning or restricting use (2018-2022).
Countries such as Germany and Norway, or individual states such Queensland in Australia, have been at the forefront in regulating the use of PFAS, especially for highly dispersive use such as firefighting foams.
Some countries are not waiting for UN decisions to regulate the use of PFAS. In Europe, PFOS has been prohibited since 2011 and PFOA since 2018; current discussions aim to stop the use of all PFAS with C4 to C20 carbons completely with a deadline of 2025, in anticipation of these restrictions many industries are moving to fluorine-free technology. In the USA, change is being driven mainly by the cost of litigation with thousands of cases against the fluorochemical industry including foam manufacturers in the pipeline.
To be continued in Part 2
