From AFFF to F3 : Chemistry – Part 2
In this part we deal with the chemistry involved in formulating Class A foams for carbonaceous fuels, and legacy Class B AFFF foams based on PFOS chemistry for liquid hydrocarbon and polar solvent fires.
In Part 1 of this series of articles we saw that AFFF firefighting foams contain various perfluoroalkyl substances (PFAS); however, firefighting foam is only one of many applications.
PFAS have been used for decades in more than 200 other industrial and domestic applications, such as food packaging, leather and textile treatment, carpet and clothing anti-stain protection, detergents, water-proofing and oil-proofing, paints and varnishes, printing inks, chromium plating, outdoor and protective clothing (PPE) for the emergency services and military. These perfluorinated substances are widely used as they offer a combination of unique properties, including the ability to repel water (hydrophobicity), the ability to repel oils (oleophobicity), the ability to reduce the surface tension of aqueous solutions to less than 20 dyne/cm and with it acting as detergents, emulsifiers, wetting agents, and dispersants.
The OECD (2021) has recently clarified the definition of what constitutes a PFAS, whilst acknowledging that given by Buck et al (2011), as follows:
“PFASs are defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS.”
More than 800 products currently available in the marketplace have been identified, but the true list of PFAS used in commerce and industry is likely to be 10,000 or more; the UN Stockholm Convention has listed 4,700 substances related to PFOA alone. PFAS started to be manufactured in large quantities in the early 50’s. All of them are anthropogenic created by humans using chemical synthesis. They do not exist naturally. Their extremely stable and chemically resistant perfluorinated end-products of breakdown in the environment have long been identified as ‘forever chemicals’, for example by scientists and journalists such as Rebecca Renner [“Growing Concern Over Perfluorinated Chemicals” (2001) Environ. Sci. Technol. 35(7) 154A-160A; “The long and the short of perfluorinated replacements” (2006) Environ. Sci. Technol. 40(1) 12-13] or Sharon Lerner writing in the Intercept [“Toxic Chemicals Discovered in Hundreds of Products” Sharon Lerner (The Intercept December 2020)].
It must be stressed that although still commonly and inaccurately referred to as ‘emerging contaminants’, PFAS have truly emerged as contaminants of concern for at least 10 years and should no longer be described as ‘emerging’. On the other hand, the technology of how to deal with PFAS waste is currently still emerging and developing.
Firefighting foams are classified either as Class A suitable for carbonaceous fuels such as wood, paper or vegetation, acting as wetting agents improving the penetration of water into deep seated fires and do not contain fluorosurfactants, only hydrocarbon surfactants; or, on the other hand, Class B foams are specifically formulated for liquid hydrocarbons such as gasoline and polar solvents such as ethanol. Modern Class B foams may either contain fluorsurfactants and be capable of film-formation at the air-fuel interface (AFFF), or completely fluorine-free, F3 foams, specially formulated containing only hydrocarbon surfactants. Interestingly Class B fluorine-free foams (F3) can be used effectively for both Class A and Class B fires unlike AFFF,
Class A foams for carbonaceous fuels
Class A firefighting foams are used extensively worldwide, especially in Australia, America and Southern Europe, for incidents involving carbonaceous fuels, e.g., structural house fires, plastic and tyre waste, as well as grassland and wildland or bush fires. Ted Schaefer then working for
3M Australia in the late 1980s developed one of the first effective Class A foams, “3M Fire-Brake BFFF”, recognised in 2001 by the Australian Academy of Technological Sciences and Engineering as one of the top 100 Australian inventions of the 20th century.
Class A foams behave very differently to fluorosurfactant-containing AFFFs, as they are specifically formulated to penetrate carbonaceous fuel effectively, such as compacted vegetation, paper or wood, using specialised hydrocarbon surfactants, not unrelated to kitchen washing-up liquid. Fluorosurfactant AFFFs, designed for surface application to liquid hydrocarbon or polar solvent fires, are nowhere nearly as efficient at penetrating such deep-seated fires and claims by some in the industry that their AFFF products can be used as dual Class A / Class B foams is frankly misleading.


Mister H: Penetration by Class A Mister F: Failure to penetrate by Class B AFFF
(Bluteau 2007)
Class B AFFF foams for liquid hydrocarbons and polar solvents
The first report of an aqueous film-forming foam (AFFF), called LightWater®, by R.L. Tuve et al of the Naval Research Laboratory and the 3M Company March 1964 of a foam capable of vapour suppression and film forming on the surface with low flash point flammable fuels such as gasoline, showed that it was 1200% more effective than standard protein foams under identical conditions.
The compounds tested in foam formulations were in the general class of perfluorosulfonic acid derivatives, some being quaternary salts, others being alcohols, esters, anionic salts of substituted sulfonamido carboxylic acids, etc. All of these water soluble, high molecular weight fluorocarbons shown dramatic surface tension depression of water to below 20 dynes/cm. In general they are insensitive to electrolytes and show surface activity when dissolved in organic solvents.
The first Patent for an AFFF was granted to Richard Tuve and Edwin Jablonski in June 1966 [1], representing a new era in firefighting foams which was to last for the next 30-40 years until the 3M Company Minnesota withdrew from PFOS-based chemistry altogether in May 2000.
Information from the patent literature gives a fascinating insight into the derivatives used in these early AFFFs. Derivatives of perfluorooctane sulfonamide (PFOSA) and perfluorooctane carboxylic acid (PFOA) were used. As reported in the 1966 patent these early formulations include the quaternary ammonium salts of PFOS and PFOA amido derivatives:
C8F17-SO2NH2-(CH3)3N(CH3)3+I-
C7F15-CONH-(CH3)3N(CH3)3+I-
an amphoteric amino betaine derivative of PFOA
C7F15-CONH-(CH2)3–N+(CH3)2-CH2-CH2-COO–
and the potassium salt of a PFOS sulphonamide derivative
C8F17SO2N(C2H5)-CH2COOK
The potassium salt of PFOS in the form of surfactant FC-95 was also used in early foams.
Interestingly it was some 50 years later that Barzen-Hanson et al in 2017 [2] from Jennifer Field’s group at Oregon State University identified a vast range of other derivatives, or their breakdown products, involving 40 different classes in legacy AFFFs.
Electrochemical Fluorination (ECF) – the Simons Process
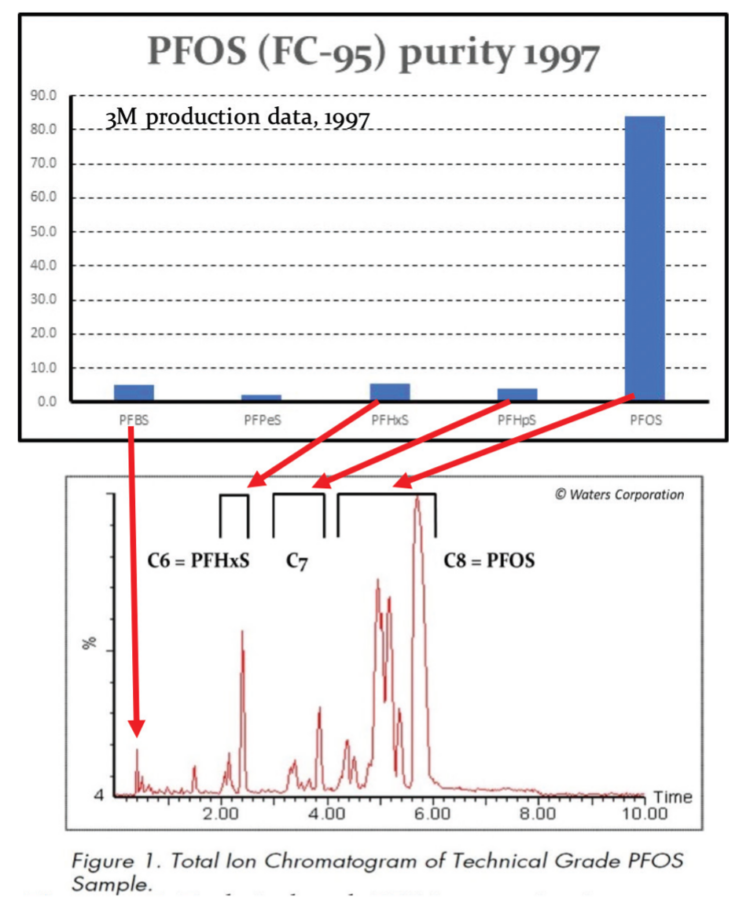
The 3M Company announced in May 2000 that it was phasing out fluorosurfactant production based on PFOS chemistry and withdrawing entirely from the fluorinated AFFF firefighting foam market marking an end to the availability of Light Water™ and Light Water™ ATC™ formulations (3M Company (2000)). Other products using PFOS included ScotchGuard™ stain and water repellent treatments. Production of PFOS by the 3M Company is thought to have ceased entirely around 2002, being replaced by the shorter chain compound PFBS, although PFOS and PFHxS production is thought to have continued in China and India.
Until 2000 PFOS had been manufactured using the Simons electrochemical fluorination (ECF) process (3M Company, 1999; Ignat’ev et al , 2009; Sartori and Ignat’ev, 1998). This process involves replacing the hydrogen atoms of octyl sulfonate using hydrogen fluoride electrolytically in order to generate perfluorooctane sulfonyl fluoride, PFOSF.
C8H17SO3H + HF ==>> C8F17(C=O)F
PFOSF is highly reactive acyl fluoride and is the starting material for preparing PFOS derivataives such as the sulfonamide PFOSA or N-ethyl-PFOSA, for example:
C8F17(C=O)F + C2H5NH2 ==>> C8F17(C=O)-NH-C2H5
PFOSF production using electro-chemical fluorination (ECF) was, and remains, an inherently ‘dirty’ process resulting in a wide range of structural isomers, both straight chain and branched with CF-CF3 and C-(CF3)2 side chains, as well as odd and even chain length homologues such as C4 PFBS, C6 PFHxS and C7 PFHpS. As a result, technical grade PFOS was always and continues to be contaminated with a significant percentage of PFHxS. In addition, the perfluoroalkyl chains of both PFOS and PFHxS can form left- or right-handed helices resulting in pseudo-racemates that have been detected in human sera (Wang et al, 2011; Naile et al , 2016; Sasaki et al , 2018). Quoting from the ECHA (13 June 2019) PFHxS restriction proposal:
“…Sources indicate that when manufacturing perfluorinated compounds, a mixture of compounds of varying chain- length is usually formed, with typical amounts of PFHxS formed when manufacturing PFOS being between 4 and 14% ( from (BiPRO, 2018) citing (Ren, 2016). These numbers are supported by measurements of PFHxS in commercial PFOS-products, namely 3.5%–9.8% in 3M’s FC-95 (from (BiPRO, 2018) citing 3M (2015) and 11.2 % – 14.2% in three products from China (Jiang et al, 2015). BiPRO also note, however, that the amount of the C6-component may be reducedby purification at different stages of the production line….”
The significance of the relatively high levels of the C6 homologue perfluorohexane sulfonic acid, PFHxS, in these AFFF formulations is that PFHxS is more toxic and bioaccumulative than PFOS, has a longer biological half-life in humans, and has also been list in the Annexes of the UN Stockholm Convention for restriction. Unfortunately, some manufacturers especially in Asia have used PFHxS as a ‘regrettable substitution’ for PFOS.
The use of ECF to produce perfluorinated sulfonic and carboxylic acids, such as PFOS and PFOA and their derivatives, has been summarised by Buck et al [2011], as shown below.
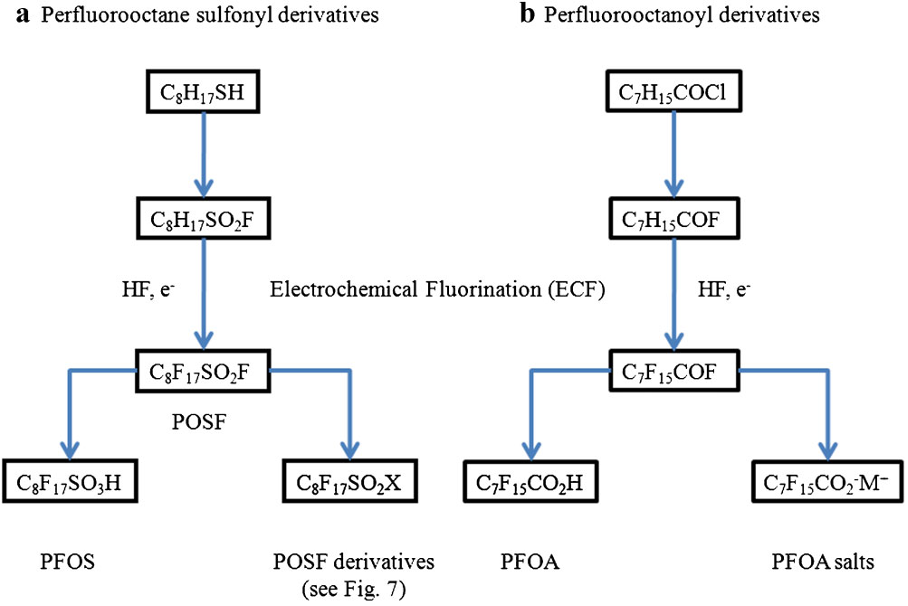
source: Buck et al (2011)
To be continued as Part3.
References
Barzen-Hanson, K.A., Roberts, S.C., Choyke, S., Oetjen, K., McAlees, A., Riddell, N., McCrindle, R., Ferguson, P.L., Higgins, C.P., and Field, J.A.. (2017) “Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater” Environ, Sci. Technol. 51, 2047-2057.
Benskin J.P., De Silva A.O., Martin J.W. (2010) Isomer Profiling of Perfluorinated Substances as a Tool for Source Tracking: A Review of Early Findings and Future Applications. Rev. Environ. Contam. Toxicol.:111-160.
Buck, R.C., Franklin, J., Berger, U., Conder, J.M., Cousins, I.T., de Voogt, P., Jensen, A.A., Kannan, K., Mabury, S.A., and van Leeuwen, S.P.J. (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 7(4), 513-541.
Ignat’ev, N.V., Willner, W., and Sartori, P. (2009) Electrochemical fluorination (Simons process) – A powerful tool for the preparation of new conducting salts, ionic liquids and strong Brǿnsted acids. J. Fluorine Chem. 130(12), 1183-1191.
Naile, J., Garrison, A.W., Avants, J.K., and Washington, J.W. Isomers/Enatiomers of Perfluorcarboxylic Acids: Method Development and Detection in Environmental Samples. Chemosphere 44, 1722-1728.
OECD (2021), Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances:
Recommendations and Practical Guidance, OECD Series on Risk Management, No. 61, OECD
Publishing, Paris.
Sartori, P. and Ignat’ev, N.V. (1988) The actual state of our knowledge about mechanism of electrochemical fluorination in anhydrous hydrogen fluoride. J. Fluorine Chem. 87(2(, 157-162.
Sasaki, T., Egami, A., Yajima, T., Uekusa, H., and Sato, H. (2018) Unusual Molecular and Supramolecular Structures of Chiral Low Molecular Weight Organogelators with Long Perfluoroalkyl Chains. Crystal Growth and Design 18(7) 4200-4205.
Tuve, R.L. amd Jablonksi, E.J. (1966) US 3,258,423 Patent June 28, 1966 “Method Of Extinguishing Liquid Hydrocarbon Fires”, assignors to the United States of America as represented by the Secretary of the Navy. Filed Sept. 4, 1963, Ser. No. 306,665.
Vyas, S.M., Kania-Korwel, I., Lehmler, H.J. (2007) Differences in isomer composition of perfluorooctanoylsulfonyl (PFOS) derivatives. J. Environ. Sci. Health and Toxic Hazard Substance Environ. Eng. 42, 249-255.
Wang, Y., Beeson, S., Benskin, J.P., De Silva, A.O., Genuis, S.J., and Martin J.W. (2011) Enantiomer Fractions of Chiral Perfluoroctanesulfonate (PFOS) in Human Sera. Environ. Sci. Technol. 45(20) 8907-8914.
