The magazine SOLDATS DU FEU organized the Thematic Days from March 19 to 21, 2025. More than 100 delegates attended the event. 3F was present at the booth of its distributor JCM for its fluorine-free foam concentrates and additives for Class A fires.
One of the topics discussed was the new fluorine-free foam concentrates known as F3. Dr. Thierry Bluteau, a chemist at LEIA Ltd, discussed the problems encountered with F3 foams, such as “excessive” viscosity, storage instability, and water sensitivity. He discussed the solutions provided by innovative manufacturers, such as 3F. The event was a resounding success and confirmed the industry’s shift toward high-performance, environmentally friendly products.
News
Thematic Days in Troyes, France, from March 19th to 21st, 2025
From AFFF to F3 : F3 comes to light — Part 6
Previous articles (Parts 1 to 5) have dealt with the history, chemistry, environmental and health impacts and regulations of Firefighting foams.
Introduction
From 3M’s phasing-out of fluorochemicals industry in 2000 to actual market, an ever-growing number of manufacturers have been introducing fluorine-free firefighting foams. If today, a manufacturer says that F3 are evidence and mandatory, It has not been like that in the early years after 2000.
The first years 2000-2012
In fact, during more than 12 years, only two chemists – namely Ted Schaeffer and Thierry Bluteau – were actively working to offer the first generation of F3, respectively Re-Healing Foam (Solberg) and ECOPOL(Bio-ex) These first F3 were relatively good on solvent fires but showed some weaknesses when applied on hydrocarbon fuels.
At the same time, a commercial battle was raging on between these two companies and the rest of the industry – grouped under the FFFC banner – to maintain or not the AFFF technology on the market. Despite all these efforts to undermine the F3 technology, the wind of change started to blow and several customers decided to change to an eco-friendly efficient foam.
The last years 2013-2023
This period can be considered as a transition time, when it became evidence that F3 would become in a short time the firefighting foam for the future and AFFF become a product from the past. This period brought awareness in both the customers and the manufacturers and in a few years appeared the second generation of F3. The main difference was about the fire performance on hydrocarbon fires, as it claimed the top rating under EN1568-3 and UL162. In the same time, the early chemists changed their position: Thierry Bluteau moved to work with 3FFF LTD and Ted Schaeffer retired.
Today one can find on the market at least 8-10 F3 foams, with variable performance. Most of the manufacturers are based in Europe and USA.
The level of performance has been brought to the highest level of the EN1568-3 and 4 standard and matches the expected performance for AFFF type foams.
The F3 technology
The chemist had to face a problem form the start: the removal of fluorinated compound from the original formulation meant that he would need a chemical compound/blend to offer and balance the same properties, to say the resistance to heat and fuel contamination.
We have seen in previous articles that synthetic foam formulation is a combination of surfactants, hydrophilic solvents and polymers. The choice is still wide for the chemist.
More than 7000 surfactants are listed and identified.
For the hydrophilic solvents, we can account between 800 and 1000 different molecules.
And for the polymers, it is again a wide choice, probably more than 1000 polymer types exist, without counting the numerous copolymers and the variations in polymer length and ramification degree.
We can then measure the difficulty for the chemist to select among a large choice of chemicals to combine and obtain the final product.
A suitable formulation for a firefighting foam, is the balance between the different components to achieve the desire level of extinction, while maintaining acceptable physical parameters, such as density, pH, viscosity, foam expansion, drainage time and stability over ageing.
To make a story short, the actual F3 are relying on a combination of surfactants and natural polymers to achieve an acceptable level of extinction.
Unfortunately, the use of an excessive quantity of polymers results in a dramatic increase of viscosity, making some foams almost impossible to be inducted with common dosing systems. Some dosing systems manufacturers had to modify their injection system to cope with this high viscosity; and in some case, some foams are just impossible to use on a practical point of view.
We have seen that, even if the best performing foam F3 can challenge the performance of AFFF, they are very viscous and need a specific – and expensive -dosing system to be used. That can result in a significant side cost which finally would impact on the global costs of the fire protection equipment.
Aside from the viscosity, most of these F3 contain a high quantity of solvents – mainly glycol and glycol ethers – with potential toxic effects. Most of these solvents can migrate through the skin and provoke allergy, skin sensitivity/irritation, and other health effects.
The F3 third generation
3F company dedicated itself since the start to produce F3s designed to fit perfectly to customer needs. Since this philosophy has always been in its DNA, 3F offers a full line of F3 – known as FREEGEN – to address all the potential request from the industry.
That is why 3F developed an exclusive know-how in foams and offers SMART FOAM ® : this technology allows the complete removal of solvents from the formulation. On top of that, 3F developed a special combination to control the viscosity of the product while maintaining a high level of performance on fire. As a result, 3F offers 2 SMART FOAM FREEGEN SF-LV and FREEGEN ULTRA. FREEGEN SF-LV can be inducted with any proportioning system on the market – including simple Venturi.
CONCLUSION
F3 technology has been proven efficient and easily available. With the support of EU regulation, the industry is already moving and replacing their old stock of AFFFs with F3s. F3 are continuously developed to offer to end-users more choice in concentration of use and performance. At the moment of making a choice, the fire chief must check that the foam complies with his needs and equipment, and meets the requirement he is looking for (LastFire, VDS, EN, ICAO,…)
From AFFF to F3 : Regulation and Legislative Pressures — Part 5
Previous articles (Parts 1 to 4) have dealt with the history, chemistry, environmental and health impacts of PFAS exposure.
Introduction
Regulators have become increasingly aware of the risks implied by the manufacture and use of perfluorinated compounds in industrial and consumer products and, as a result, many countries have enacted legislation restricting the use and disposal of PFAS.
Lack of knowledge of best available practice (BAP) or the law is no defence when it comes to defending one’s actions that may have led to environmental contamination, as encapsulated in the legal phrase “ignorantia legis neminem excusat (“ignorance of law excuses no one”)”. This doctrine first shows up in the Bible in Leviticus 5:17: “If a person sins and does what is forbidden in any of the Lord’s commands, even though he does not know it, he is guilty and will be held responsible.”
A modern interpretation of this principle has been established under the Rio Convention in 1992 as the precautionary principle under which lack of evidence must not be treated as evidence of lack of harm, especially where long term intergenerational effects may be suspected.
The REEBOK seminars
Shortly after the announcement by the 3M Company in May 2000 that they were withdrawing from PFOS-based chemistry, RAK and his colleagues arranged a series of international conferences in Manchester and Bolton to discuss environmental issues associated with firefighting foams. These Reebok seminars which took place in 2002, 2004, 2007, 2009 and 2013, were well attended by the fluorochemical industry, regulators, foam manufacturers and end-users such as fire departments worldwide. These seminars became the de facto industry forum for discussing the phase out of Class B AFFF and the transition to Class B fluorine-free foams (F3).
At the earliest Reebok seminar in 2002 it was pointed out that the U.K. GroundWater Regulations 1998 already prohibited the discharge of fluorochemicals as found in AFFF foams to groundwater if they were PBT – Persistent, Bio-accumulative and Toxic. Unfortunately given clear lack of knowledge of the significance of the term ‘organohalogen’ of the regulation (SI 2746/1998) it was claimed that this was not relevant.
Later Reebok seminars, especially in 2004 and 2007, concentrated not just on PFOS but also on PFOA and related substances that could degrade to PFOA, a typical perfluorinated extremely persistent PFCA end-product of breakdown. The possibility of derivatives such as 8:2FTS, as found in C6/C8 fluorotelomer foams, bio-degrading to yield PFOA was at first vigorously denied by the fluorochemical industry in the face of published evidence suggesting the contrary. However, the whole tenor of the discussion changed as a result of pressure from the US-EPA which resulted in the 2010-2015 PFOA Stewardship Programme leading ultimately to a requirement for fluorotelomer products to contain less than 25 ppb PFOA or its precursors.
i) 1998: Subject to sub-paragraph (2) below, a substance is in list I if it belongs to one of the following families or groups of substances–
(a) organo-halogen compounds and substances which may form such compounds in the aquatic environment;
ii) 2009: This includes in particular the following when they are toxic, persistent and liable to bio-accumulate—
(a) organohalogen compounds and substances which may form such compounds in the aquatic environment;
UBA
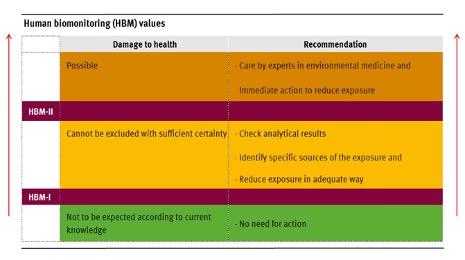
The German Federal Environment Agency’s (das Umweltbundesamt or UBA) Human Biomonitoring Commission has established trigger levels for both PFOS and PFOA in human blood plasma. The HBM-I value is the plasma concentration in ng/ml (ppb) that is estimated to pose no appreciable risk to the human population for prolonged exposure and, thus,no need for further action. Concentrations between HBM-I and HBM-II indicate that health impacts cannot be excluded with sufficient certainty and that further investigation is necessary with the introduction of measures to eliminate, reduce or control exposure, including identifying potential sources and checking analytical results. Plasma concentrations in excess of HBM-II values, indicating possible health impacts, require immediate intervention to reduce or eliminate exposure and assessment of any health impacts.
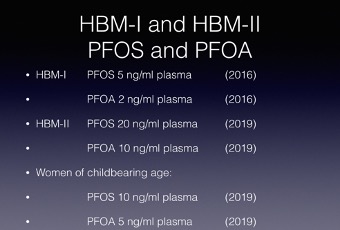
The HBM-I and HBM-II values for PFOS and PFOA are shown below. For women of child-bearing age the HBM-II values recommended are half those for the general population. It is worth noting the PFOA values are less than those for PFOS.
FIREFIGHTERS EXPOSURE
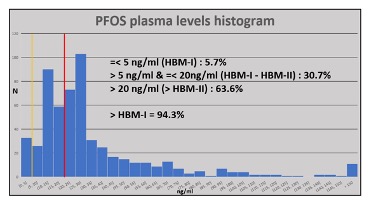
The story for firefighters who are or have been occupationally exposed to PFAS-based AFFF is a rather different story. Long service firefighters as well as those more recently employed may have plasma levels far in excess of acceptable values especially for PFOS and its homologue PFHxS, which are correlated. As an example, the figure below shows PFOS plasma levels in 2015-2016 for an extensive cohort of firefighters from a large Australian fire brigade, in which the majority had levels in excess of HBM-II. THE HBM-I level is shown as the yellow vertical line, HBM-II in red. Nearly 2/3 of the sampled cohort exceeded HBM-II indicating a potential health risk.
The major exposure pathway for firefighter exposure to PFAS is clearly the use of AFFF fluorochemical containing firefighting foam either operationally or during training and maintenance. The mechanism of exposure is undoubtedly either the inhalation of foam aerosol especially for those personnel not wearing self-contained breathing apparatus (SCBA), or through dermal absorption as a result of being soaked with foam spray. At any major incident involving foam, for example a fuel tank fire, approximately a third of the foam applied from monitors never reaches the fire itself but is lost as a foam curtain and aerosol which contaminates the surrounding area and any personnel present.

Drinking water
Drinking water is the one unavoidable exposure pathway for everyone. Average intake is calculated as ~2 litres/day over one’s lifetime. Maximum total weekly intakes (TWI) are calculated on this basis.
The revised EU Drinking Water Directive entered force in January 2021, with full compliance by 12 January 2023, and included measures tackling emerging contaminants such as endocrine disruptors and PFAS, as well as microplastics. The maximum concentration of all PFAS compounds combined 0.5 μg per liter of water (500 ppb). Alternatively, member states can monitor the sum of 20 specified PFAS compounds, for which the maximum is 0.1 μg/l (100 ppb).
The Netherlands National Institute for Public Health and the Environment (RVIM) has recently derived new limits risk limits for PFAS in surface waters [*2022-0074 RVIM 2022-09-08] based on EFSA derived health-based values for PFAS in 2020. The new risk limits are 0.3 ng/l (0.3 ppt) for PFOA, 7 pg/l (0.007 ppt) for PFOS), and 10 ng/l (10 ppt) for Gen-X. These are much lower than current standards for these PFAS.
Food
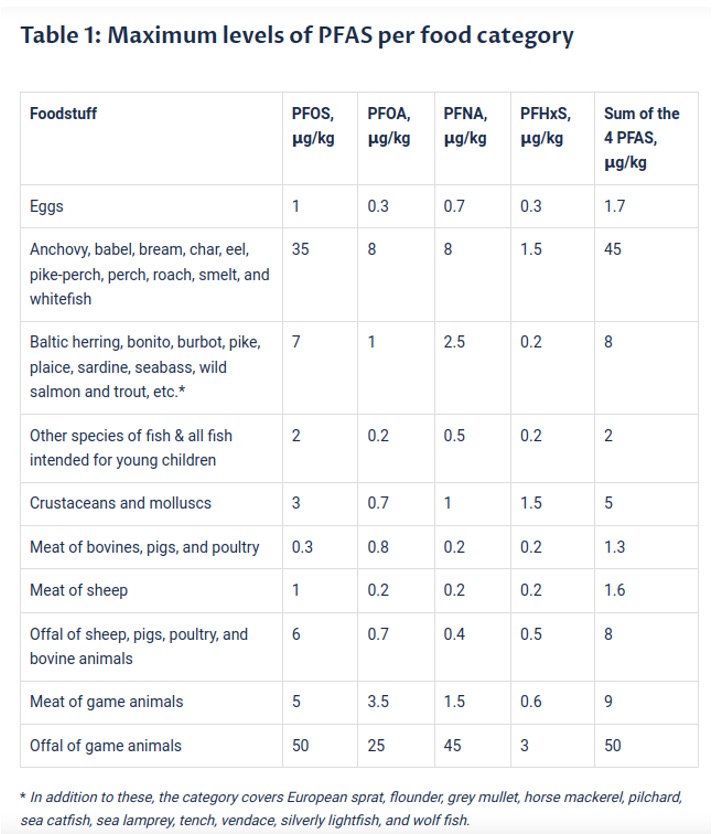
The limits on PFAS in selected food products on the EU market are listed in Table 1. If higher concentrations are discovered in laboratory tests, the product has to be removed from the market.
UN – Stockholm Convention for POP
The Intergovernmental Forum on Chemical Safety (IFCS) and the International Programme on Chemical Safety (IPCS) prepared an assessment of the 12 worst offenders, known as the dirty dozen, as the initial POPs listed under the Annexes of the Stockholm Convention. These substances included the pesticides Aldrin, Chlordane, DDT, Dieldrin, Endrin, Heptachlor, Hexachlorobenzene HCB, Mirex, Toxaphene; and the industrial chemicals polychlorinated biphenyls PCB, polychlorinated dibenzo-p-dioxins PCDD and polychlorinated dibenzofurans PCDF.
The INC met five times between June 1998 and December 2000 to elaborate the convention, and delegates adopted the Stockholm Convention on POPs at the Conference of the Plenipotentiaries convened in May 2001 in Stockholm, Sweden. The convention entered into force on 17 May 2004 with ratification by an initial 128 parties and 151 signatories. Co-signatories agreed to outlaw nine of the dirty dozen chemicals.
The first set of new chemicals to be added to the convention were agreed at a conference in Geneva on 8 May 2009.
In 2009, perfluorooctane sulfonic acid and its derivatives -PFOS- have been included in the UN Stockholm Convention to eliminate their use (decision SC-4/17). In 2019, following the evaluation by POPRC-14 in 2018 at FAO Headquarters in Rome of the continued need for PFOS, its salts and PFOSF, COP9 amended Annex B to remove several of the specific exemptions and acceptable purposes for PFOS, its salts and PFOSF (decision SC-9/4).
• UNEP/POPS/POPRC.2/17/Add.5: Risk profile for perfluorooctane sulfonate
• UNEP/POPS/POPRC.3/20/Add.5: Risk management evaluation for perfluorooctane sulfonate
• UNEP/POPS/POPRC.4/15/Add.6: Addendum to the risk management evaluation for perfluorooctane sulfonate
In 2019, COP9 listed PFOA and its derivates in Annex A to the Stockholm Convention (decision SC-9/12). POPRC Secretariat developed an indicative list of substances, originally containing around 4,700 compounds; the list is to be updated periodically. PFOA has been banned under the POPs Regulation since 4 July 2020.
• UNEP/POPS/POPRC.12/11/Add.2: Risk profile for PFOA, its salts and PFOA-related compounds
• UNEP/POPS/POPRC.12/INF/5: Additional information relating to the risk profile for PFOA, its salts and PFOA-related compounds
• UNEP/POPS/POPRC.13/7/Add.2: Risk management evaluation on PFOA, its salts and PFOA-related compounds
• UNEP/POPS/POPRC.14/6/Add.2: Addendum to the risk management evaluation on PFOA, its salts and PFOA-related compounds
• UNEP/POPS/POPRC.17/INF/14/Rev.1: Updated indicative list of substances covered by the listing of PFOA, its salts and PFOA-related compounds
In June 2022, the UN Stockholm Convention also decided to include PFHxS and related compounds in the treaty. The Commission added the substance group in the EU’s POPs Regulation in May 2023 and the regulation entered into force on 28 August 2023.
The POPRC is currently reviewing LongChain-PFCAs, and related compounds, proposed for listing in Annexes to the Stockholm Convention.
• UNEP/POPS/POPRC.17/7: Proposal to list long-chain perfluorocarboxylic acids, their salts and related compounds in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants
White Papers from IPEN PFAS Expert Panel 2018-2019
Three influential White Papers produced by the IPEN PFAS Expert Panel in 2018-2019, coordinated by Roger Klein and colleagues, were presented to the UN Stockholm Convention’s POPRC14 in 2018, COP9 and POPRC15 in 2019 discussing progress in transitioning from AFFF to F3 firefighting foams, issues associated with PFAS and PFHxS. These White Papers assisted the Committee in achieving the listing of these PFAS under the appropriate Annexes for restriction or banning in 2019 and 2022.
As of September 2022, there are 186 parties to the UN Stockholm Convention (185 states and the European Union). Notable non-ratifying states include the United States, Israel, and Malaysia.
EU Regulation
The first product to be regulated has been PFOS.
Norway prohibited the use of PFOS-containing materials in 2007.
The European Union Commission Regulation (No. 757/2010) required that all foam containing PFOS above 10 mg/kg (0.001% w/w or 10 ppm) must not be used after 27 June 2011 and this was adopted by the UK Environment Agency in February 2011.
In January 2014, the Environmental Agency of Norway has published the Regulation FOR-2013-05-27-550, which bans the use of PerfluroOctanoic Acid -PFOA- and its salts and esters.
Limits were implemented on July 1, 2014 to all products manufactured, imported, exported and marketed in Norway, with the exception of some specific articles for which the new rules were applied in January 1, 2016.
In 2020, EU 2020/784 regulates the use of PFOA and limits the content to 25ppb. In theory, it can be used until 2025 if all effluents can be contained, which is impossible to guarantee. In the facts, the regulation eliminates PFOA in Europe.
Long Chain Perfluorinated carboxylic acids (C9-14 PFCAs), their salts and precursors are restricted in the EU/EEA – EU Regulation 2021/1297 -from February 2023 onwards.
Germany – supported by Sweden, Netherlands, Denmark and Norway – has proposed a further restriction for PerFluoroHexanoic Acid C6 -PFHxA-, its salts and related substances. This proposal is highly significant as it would effectively ban the use of C6-fluorotelomer AFFF foams. This proposal was evaluated by ECHA in December 2021.
The European Chemical Agency -ECHA- is evaluating a global restriction on an EU-wide PFAS ban on firefighting foams. The restriction could reduce PFAS emissions into the environment by around 13 200 tons over 30 years [*June 2023 ECHA/NR/23/19]. An answer is expected to be given in January 2024.
Australia
Until recently, there has not been a Federal approach to regulate the use of AFFF in Australia; but each sate had its own way to restrict the use of fluorinated foams.
Canberra – Federal – published in May 2020 NEMP 2.0 to establish a practical basis for nationally consistent environmental guidance and standards for managing PFAS contamination. A draft of PFAS in NEMP 3.0 was released in September 2022.
Queensland
Queensland Fire Service is using F3 foam since 2003
Queensland Environment Authorities controls PFAS content of AFFF products and banned PFOS.
New South Wales
Fire Services had been using 3M’s foams; and stopped their use in 2007. The Protection of the Environment Operations Regulation 2022 bans and restricts the use of PFAS firefighting foam in NSW to reduce its impact on the environment, while still allowing its use for preventing or fighting catastrophic fires by relevant authorities and exempt entities. The Regulation aligns with the National PFAS Position Statement and is the first step to achieving the agreed objectives in the Statement.
Victoria
In 2007, Victoria Fire Service – MFB- made a decision to replace existing firefighting foam with fluorine-free firefighting foam. This decision was made on the basis of concerns relating to firefighter health and environmental issues. MFB developed an ‘Operational Use of Firefighting Foam Policy’ which was formally endorsed by the Environmental Protection Authority (EPA), WorkSafe and the Country Fire Authority (CFA). During 2011, based on independent scientific studies into PFAS, that identified links to various cancers and other health concerns, MFB extensively trialled and Fire Rescue Victoria evaluated various fluorine-free firefighting foam in hot fire, flammable liquid, B Class fire scenarios. MFB found that the fluorine-free foam consistently performed well in extinguishing B Class fires and provided MFB firefighters with a proven ‘safer’ alternative extinguishing medium. This work provided MFB with an operational firefighting foam solution that could be effectively used at Department of Defence sites, such as RAAF Airbases at Point Cook and Laverton. This enables MFB and FRV to meet its obligations for the delivery of emergency services to Defence bases using firefighting foam that does not contain PFAS. By 2014, all MFB firefighting appliances had been converted to only carry fluorine free B Class foam in their foam tanks.
South Australia
A ban on fluorinated foams came into effect in South Australia in January 2018, but licensees have the opportunity to apply for an exemption in certain circumstances.
Western Australia
Outlined work has been undertaken to identify and manage PFAS in December 2017; but now following Federal NEMP.
Tasmania
TasPorts is a leader in Tasmania’s approach to managing PFAS and fully eliminated all PFAS-containing foams from the island.
AirServices
Since 2010, Airservices has carried PFAS-free fire fighting foam at all civilian airports where it operates.
New Zealand
New Zealand excluded PFOS and PFOA from use in any solid or liquid substances that are imported or manufactured for use as a firefighting chemical in the Fire Fighting Chemicals Group Standard 2006 under the NZ Hazardous Substances and New Organisms Act 1996.
Firefighting foam soil and water contamination has been reviewed in December 2020. Total ban of PFAS in firefighting foams will apply from December 2025.
Food Standards Australia & New Zealand (FSANZ) launched a monitoring of Perfluorinated Chemicals in Food since April 2017, with Health guidance for Daily Intake Dosis values for PFOS, PFOA and PFHxS.
Canada
Canada has always been at the forefront of awareness and regulation as regards the inherent and potential risks of releasing PFAS to the environment and exposing the human population to PFAS contamination through drinking water, agricultural products and domestic use.
The Government of Canada is considering activities that would address PFAS as a class rather than as individual substances or in smaller groups. Addressing PFAS as a class of chemicals would reduce the chance of regrettable substitution, support more holistic research and monitoring programs, and provide an opportunity for a decrease of future environmental and human exposure to PFAS. A notice of intent to address the broad class of PFAS was published in the Canada Gazette, Part I: Vol. 155 No. 17 – April 24, 2021.
In response to the commitment described in the notice of intent the Government of Canada has published a Draft State of Per- and polyfluoroalkyl substances (PFAS) Report for a 60-day public comment. The related notice was published in the Canada Gazette, Part I: Vol. 157, No. 20 – May 20, 2023.
Currently, only a limited number of PFAS subgroups are subject to regulation in Canada. PFOS, PFOA, LC-PFCAs and related derivates have been assessed and added to the List of Toxic Substances under Schedule 1 of the Canadian Environmental Protection Act, 1999 (CEPA).
Since 2016, the manufacture, use, sale, offer for sale or import of PFOS, PFOA, LC-PFCAs, and products that contain them have been prohibited, with a limited number of exemptions under the Prohibition of Certain Toxic Substances Regulations, 2012. In May 2022, the federal government published a proposed new Prohibition of Certain Toxic Substances Regulations, 2022, which would replace the Prohibition of Certain Toxic Substances Regulations, 2012 and eliminate most exemptions allowing the use, sale, or import of PFOS, PFOA and LC-PFCAs in Canada.
Canada prohibited the use of foam containing PFOS above 0.5 ppm in May 2013, the regulation came into force in May 2008.
United States of America
In 2004, it was estimated that there was approximately 45 million liters of AFFF concentrate in the United States and its territories.
The National Defense Authorization Act passed by Congress in late 2019, required the US Navy to publish a new military specification for a fluorine-free foam by the end of January 2023 and required a full transition to fluorine-free firefighting foams by October 2024. Congress had imposed earlier mandates on the FAA to transition airports to fluorine-free foams by October 2021, but that deadline was missed. The new MIL-SPEC regulations pave the way for both FAA airports and military bases to transition, requiring significant investment. According to a 2021 Congressional report, the Military and Civilian airports still have about ~50 million litres of AFFF concentrate at its facilities. Although the military has yet to officially approve a f1uorine-free foam for use at its facilities, there now exist a number of commercially available products that have passed appropriate performance tests such as UL.
In the USA, every state has been going its separate way. The scandal of withheld fluorochemical toxicity and environmental impact information as well as outright misinformation, emerged from a long series of judicial court cases against PFAS feedstock manufacturers. The story even culminated in the publication of advertisements from attorneys looking for customers to go to court!

State governments are taking legislative and regulatory actions to phase out PFAS in products to prevent contamination in favour of safer alternatives. For example, laws in ME and WA have given state agencies authority to ban PFAS in a wide range of products. Maine’s law requires product manufacturers to disclose the presence of PFAS. Several states have adopted restrictions on PFAS in textiles with CA banning PFAS in almost all textiles by 2025, and NY restricting them in apparel, CO banning them in upholstered furniture, and WA moving forward on regulatory actions on many categories of textile products. Six states (CA, CO, ME, MD, NY, and VT) have adopted restrictions on PFAS in carpets, rugs and after-market textile treatments. Twelve states (CA, CO, CT, HI, MD, ME, MN, NY, OR, RI, VT, and WA) have enacted state bans on PFAS in food packaging. Four states (CA, CO, MD, and WA) have adopted restrictions on PFAS in personal care products. CO also adopted restrictions on oil and gas products. Eleven states including CA, CO, CT, HI, IL, ME, MD, NH, NY, VT, and WA have put in place bans on the sale of firefighting foam containing PFAS. With legislation adopted last year, WA is evaluating safer alternatives for PFAS in other products such as apparel, cleaners, coatings and floor finishes, firefighter turnout gear and others with a timeline of adopting restrictions by 2025. Source www.saferstates.org
Due to the judicial court cases, most of the national manufacturers ceased the production of AFFF foam in 2024 and started to promote fluorine-free foams, not only in the USA, but to all their traditional markets and customers.
Disposal of PFAS waste
This is huge and potentially extremely expensive problem. At major incidents such as Milford Haven (August 1983), Sandoz Basel (November 1986), Coode Island (Melbourne August 1991), , Buncefield (December 2005) or Campbellfield (Melbourne April 2019), tens of millions of liters of foam-contaminated firewater run-off were released to the environment – total containment at such large incidents is almost impossible and any waste that is collected must be disposed of appropriately. Legacy stocks of fluorinated foam concentrates represent a large financial expenditure as part of transitioning to fluorine-free products, as does the decontamination of fixed and mobile equipment.
Currently the most effective, financially feasible and environmentally sustainable method for disposing of large quantities of PFAS contaminated solid or liquid waste appears to be very high temperature incineration. Costs can range between 1000-3000 USD, strongly depending on the accepted level of residual fluorinated contaminant.
Summary
Evermore regulations, especially in Europe and Australia/New Zealand, are targeting especially dispersive applications using PFAS such as firefighting foams. The transition from AFFF to fluorine-free foams -F3- has become possible, given rapid technological advances in the last 5-10 years and fluorine-free products able to compete with AFFF based on performance.
Many large organizations such as civilian and military airfields, as well as Oil& Gas, Chemical, Mechanical industry such as Equinor, Bayer, Lufthansa and BMW, have already gone fluorine-free, having solved the problems of old AFFF equipment and appliance decontamination as well as compatibility in terms of delivery systems and operational training.
Most recently, we have seen some awareness growing in Environment Regulation Bodies – Singapore, Mexico, Colombia – showing a deep interest in what has been happening in other countries; it is likely that the ban on ‘’Forever chemicals’’ will be extended to the world community in the next 5 to 10 years !
References
Krogerus, M. (2012) The Decision Book: Fifty Models for Strategic Thinking. Tschäppeler, R. and Piening, J. (1st American edition), New York, W.W. Norton & Co., pp. 86-87.
Rumsfeld, D. (2002)
Rio Convention (1992)
United States (1933) Securities Act.
Preston, B.J. (2017) The Judicial Development of the Precautionary Principle. Queensland Government Environmental Management of Firefighting Foam Policy Implementation Seminar 21 February 2017, Brisbane, Qld., pp. 26.
Preston, B.J. (2018) ‘The Judicial Development of the Precautionary Principle’ Environmental and Planning Law Journal 135, 23-42.
Allcorn, M., T. Bluteau, J. Corfield, G. Day, M. Cornelsen, N.J.C. Holmes, R.A. Klein, J.G. McDowall, K.T. Olsen, N. Ramsden, I. Ross, T.H. Schaefer, R. Weber, K. Whitehead. (2018) “Fluorine-Free Firefighting Foams (3F) Viable Alternatives to Fluorinated Aqueous Film-Forming Foams (AFFF).” UN Stockholm Convention POPRC14 Rome 17-21 September 2018, IPEN Gothenburg Sweden: IPEN F3 Expert Panel. < www.ipen.org >
Bluteau, T., M. Cornelsen, G. Day, N.J.C. Holmes, R.A. Klein, J.G. McDowall, K.T. Olsen, M. Tisbury, and L. Ystanes. (2019) “The global pfas problem: fluorine-free alternatives as solutions firefighting foams and other sources-going fluorine-free.” UN Stockhom Convention COP9 Geneva Apr-May 2019, IPEN Gothenburg Sweden: IPEN F3 Expert Panel. < www.ipen.org >
Bluteau, T., M. Cornelsen, N.J.C. Holmes, R.A. Klein, M. Tisbury, and K. Whitehead. (2019) “Perfluorohexane sulfonate (pfhxs)-socio-economic impact, exposure, and the precautionary principle.” UN Stockholm Convention POPRC15 Rome Sep-Oct 2019, IPEN Gothenburg Sweden: IPEN F3 Expert Panel.
< www.ipen.org >
3F AMERICAS HAS SEEN THE LIGHT
3F AMERICAS will start to promote and distribute an innovative set of new products through its network of distributors in Latino America. These new products are a revolution in the field of protection of personal firefighting firemen.
One set of equipment is a luminous light line which works 2-Way. The line is highly resistant to heat, to stress and is offered in 3 versions: a personal line 25 meters, a portable line 50 meters, and a waterproof line 25 meters for underwater operations.
The other equipment is a lifesaving personal alarm. It offers several UNIQUE benefits.
It works under 6 different modes, set a visual and audible – 98 dB – alarm for an exposure to heat, impacts, movement . It is water-protected IP68 and the battery stands up to 20 hours and can be charged directly on its charging cradle. It is the lightest tool on the market and weighs only 120 g. See more …..
From AFFF to FF3 : Impact — Part 4
As detailed previously in first three parts of this series of articles, we have seen that the perfluorochemicals have a long history spanning more than 50 years and are far more complex than just considering PFOS, PFHxS and PFOA. Rather there are literally thousands of perfluorochemicals used in industry and commerce and focusing solely on a few named structures is unhelpful and a form of ‘tunnel vision’.

Currently there have been strong arguments put forward to treat PFAS as a class of chemical compounds, rather than as individual compounds each requiring the assessment of risk [Kwiatkowski et al (2020)], as a means of countering the disadvantages of concentrating on a few named PFAS. In seeking to regulate PFAS use the concept of ‘essential uses’ has been proposed by the Cousins group [Roy et al. (2022); Figuiére et al. (2023)].
Independent academic and regulatory interest in this class of compounds was virtually non-existent, with some notable exceptions, until the 3M Company announced in May 2000 that it was phasing-out all PFOS-based chemistry replacing it with PFBS chemistry (perfluorobutane sulfonic acid, the C4 homologue of PFOS). A notable exception which was highly relevant to understanding environmental contamination caused by AFFF foams was work published by Jennifer Field and her colleagues at Oregon State University around the same time or shortly after 3M announced its withdrawal from PFOS chemistry and foam manufacture [Moody et al. (2000); Schultz et al. (2004)].
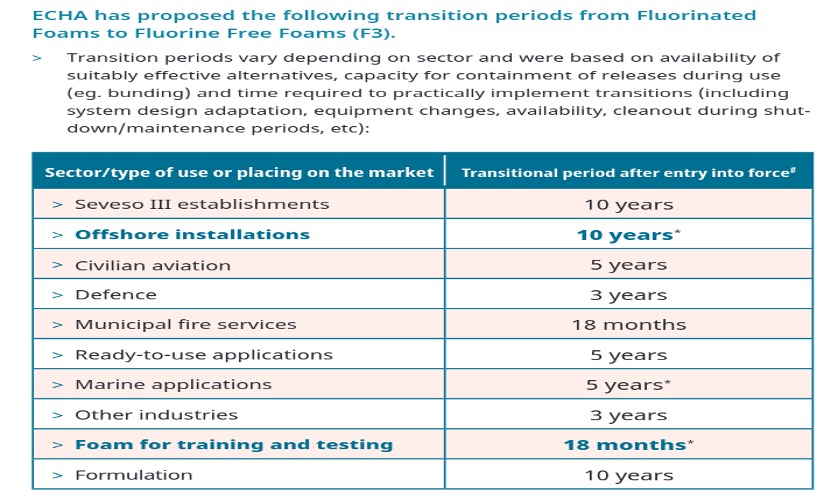
Investigative activity concerning this class of compounds started shortly after the 3M announcement in May 2000. At first most of the contributions were from the industry itself or from groups funded by the industry. It was in the late 2000s that the independent scientific community realized that there were far more that needed investigating about the impact of PFAS both on human health and the environment, with the result that the number of peer-reviewed publications increased exponentially..
Publications concerned with PFAS in the run-up to May 2000 were only in the range of 5-10 per year. After May 2000 these increased to around 50 per year and have increased exponentially since now reaching ~1000/year, frequently associated with a parallel increase in articles on microplastics [Bakhshoodeh and Santos (2022)], from whom Figure 2(b) is taken.
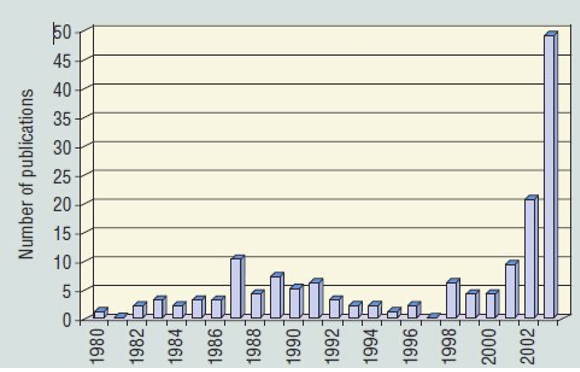
Published studies on the scale of environmental by PFOS and PFOA were scarce before 2001, until Giesy and Kannan [Giesy and Kannan (2001) and subsequent papers] reported global presence of PFOS in wildlife, with Hansen et al. finding PFOS and PFOA downstream from a manufacturing facility on the Tennessee River [Hansen et al. (2002)], as well as in biological matrices using mass spectrometry [(Hansen et al. (2001)].
Although contamination of human blood samples with organofluorine chemicals not present in stored blood taken before fluorochemical manufacturing began had been reported some years earlier since the 1960s [Taves, D.R. (1966, 1968)] with Guy et al. (1976) reporting the presence of fluorochemicals in human plasma using NMR spectroscopy, tentatively identifying PFOA as the fluorochemical used in Scotchgard® as blood contaminant [Guy, W.S. et al. (1976)]. Although there was initial confusion whether this fluorochemical was indeed PFOA or PFOS, as a result of obfuscation and a refusal to identify PFOS by 3M, it was not until 25 years later that Hansen et al. (2001) reported that samples of human plasma contained PFOS (average 28.4 ng/ml), PFHxS (average 6.6 ng/ml) and PFOA (average 6.4 ng/ml), confirming Guy and Taves findings.
A lawsuit filed in 2010 by the Attorney General of the State of Minnesota against the 3M Company revealed that the company knew these chemicals were accumulated in human blood for more than 40 years and were toxic [Lerner (2018); Swanson (2019)].
Until about 2004 the scientific literature was dominated by papers from authors working directly for 3M or funded by the industry. Post 2004 there has been an exponential explosion of independent published work concerned with PFOS, PFOA and other PFAS, to the extent that these organofluorine compounds have been labelled ‘emerging contaminants’. PFAS have emerged as contaminants of concern for at least a decade. The current situation is that although we now know a considerable amount about the environmental distribution, fate and toxicity to biota of PFAS, it is the technology for the remediation and disposal of these highly persistent materials that should be regarded as ‘emerging science’.
As said before, we are not only talking of a few substances, but about a whole family of at least 6000 different perfluorocompounds which have been classified for at least one environmental, human health and/or physicochemical endpoint in the ECHA database. At the UN Stockholm Convention Persistent Organic Pollutants Review Committee meeting in Rome (POPRC-14) an indicative list of PFOA-related substances contained 4,700 entries.
Human health endpoints are considered of major importance for long-term exposure: carcinogenicity ©; mutagenicity (M0; reproductive toxicity (R); lactation effects (L) and specific organ toxicity (STOT). 388 PFAS have at least one of these five endpoints, of which 44 are registered in harmonized classification.
With regards to environmental hazards, 1129 PFAS have been registered by self-classification; most of them counting as both (M) mobile and/or very persistent (vP).
Under the EU chemicals legislation (REACH and ECHA) the risks posed by PFAS are classified using the PBT system indicating persistence (P or vP), bio-accumualation (B or vB), and toxicity (T). Recent proposals from the German Federal Environment Agency (UBA) also stress the importance of mobility (M or vM) especially for PFAS in view of their vP and vM properties [Arp et al. (2023)]. Chemicals may also be identified as Substances of Very High Concern (SVHC) under REACH EC1907/2006 if they have serious and often irreversible effects on human health or the environment. A recent example is perfluorononanoic acid or PFNA, a PFAS that is becoming more commonly found as a contaminant. In Europe there has been far more concern with individual components of the PBT classification whereas in the United States persistence on its own with accompanying toxicity is seen as less of a problem.
Persistence
PFAS or their perfluorinated degradation products are among the most chemically and physically stable organic compounds known. Their perfluorinated carbon chains resist environmental and metabolic degradation due to the very stable C-F bonds. For example, tetrafluoromethane, CF4 and the simple perfluorocarbon, has an estimated atmospheric half-life of ~50,000 years with a high global warming potential (GW) [Mühle et al.(2010)].
Commercially available perfluoro compounds are designed to degrade rapidly once released into the environment yielding PFCAs, PFECAs and PFSAs. Unfortunately, this has led in the past to totally spurious and misleading claims made by the industry especially in the United States that these materials are ‘biodegradable’ based on degradation of the non-fluorinated functional group and the OECD rule that a substance if ‘readily biodegradable’ if degradation reaches 60% (OECD 301B, D and F) or 70% (OECD301A and E) within 28 days. This does not mean that the PFAS, for example in firefighting AFFF, is completely biodegradable as the COD method using acid dichromate for the oxidation measurement to give the 100% level totally fails to account for any perfluorinated material present. Regrettably end-users have often assumed or been led to believe by salesmen, and perpetrated as a myth by certain manufacturer [Swanson, 2019], that ‘readily biodegradable’ means total degradation. As pointed out earlier it makes sense, however, to group all PFAS as non-degradable according to their stable degradation end-products.
Breakdown of the precursors often leads to the formation of PFAS intermediates and ultimate degradation products with increased mobility in water and/or air via oxidative chemical and biochemical degradation processes in the environment.
Lifetimes of the PFAS in the environment greatly exceed the criteria for very persistent (vP) substances in Annex XIII to REACH. If PFAS do degrade, they do it so slowly that it is not observable in standard tests. The extreme persistence of PFAS and their continued use leads to sustained exposure and increasing concentrations in all environment compartments. PFASs will remain in the environment for very long time, even if releases are minimized. Increasing or legacy contamination of the environment will increase the likelihood that known and unknown effects will occur on a generational timescale. This should invoke the application of the Precautionary Principle [Rio 1992; Preston, 2017] to any further dispersive use of PFAS.
Scientists pointed out in the Helsingør Statement on PFASs [Scheringer et al., 2014] as well as in the Madrid statement [Blum et al., 2015] that very high persistence on its own presents a problem and have named this the “P-sufficient approach” to regulatory action. Persistence alone justified the regulation of PFAS as a class in California [Balan et al., 2021].
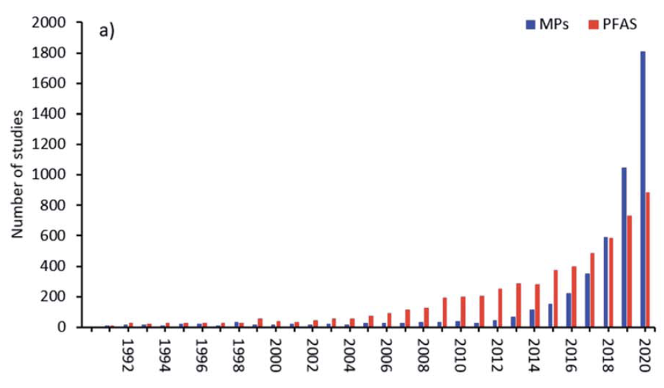
Long range transport potential (LRTP)
PFAS can be transported by air, water and matrices to which they are adsorbed or absorbed, such as dust, sediments, marine aerosols, oceanic currents, atmospheric, migratory animals, or through matrices in which they are included as additive such as microplastics. Because of their remarkable resistance to degradation, this leads to global dispersion of PFAS over long distances from the point of release. It has been estimated, for example, that for volatile PFAS such as fluorotelomer alcohols in the upper atmosphere that global circulation times can be short as 7-10 days. The historical spread through LRTP of PFAS through deposition and contamination in the Arctic is well documented [Wilson et al. (AMAP) Secretariat, 2017].
The Inuit population in the Arctic has been reported as being some of the most contaminated humans on the planet as the PFAS concentrations in their blood is much higher than the average value for the general population. Being so far removed from any industrial source of PFAS, this contamination has been attributed mainly to their diet, which based on fish, polar bear and seal meat, with an impact on the immune response [Sonne et al., 2023].
Thus, PFAS contamination of the environment and biota is not limited geographically just to the source of the pollution but becomes widespread on a global scale due to dispersive use such as in firefighting foams, poor waste disposal techniques or industrial production, compounded by long-range transport in the atmosphere and oceans.
Mobility
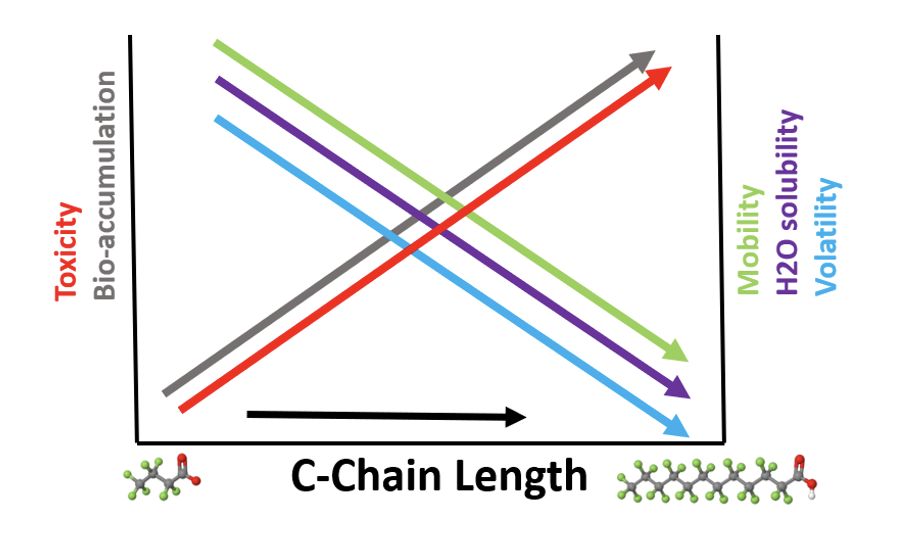
It is generally considered that substances with moderate to high solubility in water associated with low adsorption potential have a high mobility in the aqueous environment. Various studies have shown that PFAS have a different behavior depending strongly on carbon chain length and on functionality.
As shown in the figure below shorter chain-length PFAS are associated with higher environmental mobility, water solubility and volatility, as well as lower toxicity and bio-accumulation potential than longer chain-length PFAS. The combination of extreme persistence and high mobility in the aqueous compartment and soils, especially for the shorter chain PFAS such as PFPeS and PFBS, leads to contamination of drinking water aquifers and rivers as well as uptake into the food chain – fish, plants and livestock.
Accumulation in plants
A recent review article on exposure routes, bio-accumulation and toxic effects of PFASs on plants shows that bio-accumulation processes of PFASs in plants highly vary because of the complexity of PFAS chemistry [Li et al., 2022].
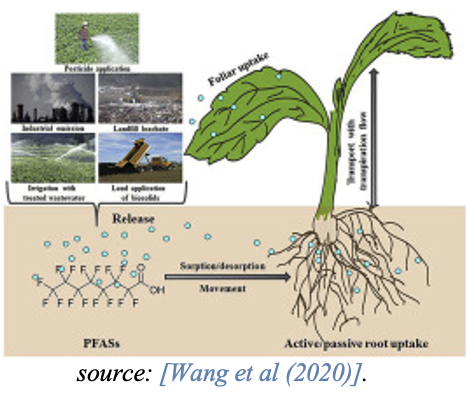
Whereas short-chain PFASs typically accumulate in above-ground plant parts such as leaves, long-chain PFASs accumulate in roots and show lower translocation factors to the above-ground plant parts. This is influenced by the higher water solubility, lower molecular size and lower hydrophobicity of the short-chain PFASs. Studies also indicate that the short-chain PFCA are more effectively taken up by plants than the long-chain PFCA [Felizeter et al., 2014; Yoo et al., 2011].
Consumption of plant material, e.g., grains and vegetables either as roots or above ground plant parts such as leaves or stems, function as a source of PFAS for both humans and animals.
Bio-accumulation and Bio-magnification (tropic magnification)
Under REACH, C11-C14 PFCAs and C6-PFSA have been shown to fulfil the vB-criterion and C8-C10-PFCA the B criterion.
Studies with mammalian species show that PFASs are readily absorbed and distributed across various tissues and that some PFAS (particularly the long-chain PFAS) have long half-lives in organisms, especially in humans where half-lives are of the order of years. Studies show that PFAS binding to albumin and transporter proteins efficiently distributes PFASs into different tissues, and enhances passage across both brain and placental barriers, with transfer to neonates via breast milk. Because of their hydrophobic and oleophobic properties PFAS do not follow typical accumulation patterns, like partitioning into adipose tissue, but rather bind and accumulate in protein-rich organs like liver.
PFASs accumulate more in air-breathing organisms as compared to gill breathing- organisms, because unlike the latter, air-breathers cannot readily eliminate PFAS by passive diffusion. Thus, established methods of bio-accumulation testing in aquatic organisms do not function adequately as methodology for PFAS bio-accumulation assessments in air-breathing species such as man, Unfortunately, laboratory bio-accumulation data are very limited for air-breathers.
Short-chain PFASs are more readily excreted by urinary excretion in air-breathing organisms and tend to be less bio-accumulative, while bio-accumulation potential usually increases with perfluoroalkyl chain length. In general, BCFs and BAFs of PFASs with 8 or more carbons increase uniformly with increasing number of carbons in the alkyl chain, with highest bio-accumulation potential seen for compounds with 12 to 14 carbon-chain length.
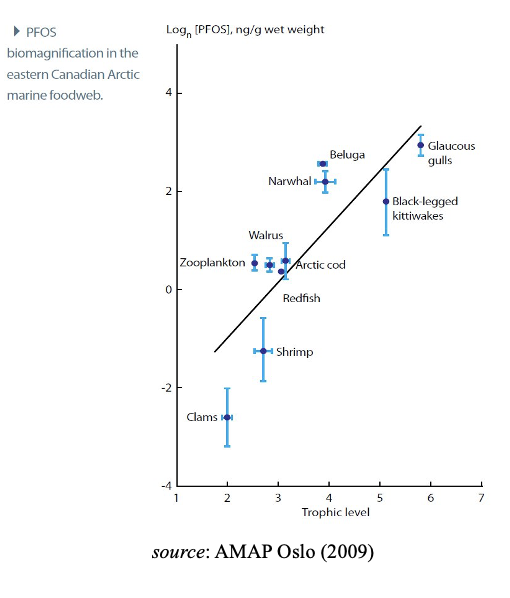
Due to these properties, many PFASs accumulate in air-breathers, and long- chain PFASs bio-magnify in marine and fresh-water food webs, reaching high levels in top predators including humans and vulnerable species. This increase in contamination seen as one ascends the food chain is known as trophic magnification and is well established for aquatic species and predators that feed off them. It is noted that consequently this may negatively affect the recommendations related to consumption of meat and/or entrails of certain animals. A a top marine predator guillemot eggs are particularly high in PFAS.Field studies on long- and short-chain PFASs that can be analytically distinguished demonstrate that PFAS
(primarily PFBA, PFBS, PFHpA, PFHxA, PFHxS, PFOS, FOSA, 6:2 FTOH, F-53B, 6:2 Cl-PFESA, TFA, and C9-C11 PFCAs) are found globally throughout the environment in mammals, birds, fish and other vertebrates. In conclusion, and considering the increasing lines of evidence from modelling, laboratory and monitoring studies, there is a justifiable rising level of concern for a subset of PFAS being bio-accumulative while large uncertainties remain for the majority of compounds due to lack of data.
Effects on human health
A vast amount of literature has been published on the health effects of PFAS, especially for PFOA and PFOS. In humans, many perfluoroalkyl acids (PFAA) are readily absorbed by inhalation or ingestion orally, while less is known regarding absorption after dermal exposure. Many PFAA bind to proteins and are thus distributed to protein-rich tissues including liver, kidneys, and blood. Estimated human elimination half-lives for PFAAs range from a few days (PFBA) and months (PFHxA, PFBS) to a few (2-8) years (PFOA, PFNA, PFDA, PFHxS, PFOS), or to >10 years for PFUnDA. Half-lives are much shorter in rodents than in humans and differences in half-lives between sexes is often observed. Consequently, the observed toxicity in rodents underestimates the toxicity to humans. PFAA are mainly excreted via urine and faeces and thus are released to the environment. PFAA have a marked potential for bio-accumulation in humans as shown by the long half-lives and protein-binding.
The European Food Standards Agency (EFSA) extensively reviewed the epidemiological evidence for association between PFAS exposure and adverse effects in humans [EFSA, 2018; EFSA, 2020]. EFSA concluded that increased serum levels of various PFCA and PFSA provoked a reduction in the immune response to vaccination [Grandjean, 2012], increased propensity of infections, increased serum cholesterol, increased serum alanine transferase (ALT) and reduced birth weight. The association with immune effects was considered the most sensitive endpoint in humans (supported by data from experimental animals) and based on this EFSA has established a Tolerable Weekly Intake (TWI) of 4.4 ng/kg body weight/week for the sum of PFOA, PFOS, PFNA and PFHxS [EFSA, 2020].
Experimental animal studies across different groups of PFAS demonstrate that liver, kidney, thyroid, the immune system, and reproduction are major targets for PFAAs’ toxicity. In rat studies, the most consistent effects included enlarged liver, hepatocellular hypertrophy, increased serum ALT, increased kidney weight, reprotoxicity, effects on the lymph system, and decreased serum thyroid hormone levels. In particular liver effects have been observed for most PFAA for which animal studies are available. For PFOS, PFOA, PFNA, and PFDA and their salts this has resulted in harmonized classifications for carcinogenicity (Carc. 2), reproductive toxicity (Repr. 1B), lactation effects (Lact.) and specific target organ toxicity – repeated exposure (STOT RE 1, except for PFDA).
Cumulative effects of co-occurring PFAS
Many different PFAS co-occur in the environment, drinking water, food, and in human blood. Many PFAS exhibit similar effects, such as effects on the liver, kidney, thyroid, serum lipids, and immune system. Accordingly, an assessment of hazards and risks taking into account such combined exposure would reflect exposure conditions more realistically than single compound assessments.
Due to the immense number of PFAS and the lack of toxicological data for the vast majority of them, a combined assessment for all PFASs is unattainable. It is emphasized at this point that combined exposure to different PFASs affecting the same target organs may result in combined more than additive effects, i.e., synergism, making the exceeding of thresholds or limit values more likely than for assessment for individual substances on their own.
CONCLUSION
PFAS are very Persistent (vP) and many are also very Bioaccumulative (vB). Long-range transport processes (LRTP) results in planetary contamination including remote regions such as the Arctic.
Cousins et al. have recently introduced the concept of having already exceeded the ‘planetary boundary’ indicating that global environmental concentrations for PFAS already exceed tolerable sustainable levels [Cousins et al., 2022]. This should be treated as a warning against continuing use and release of PFAS to the environment, especially from dispersive applications such as AFFF firefighting foam.
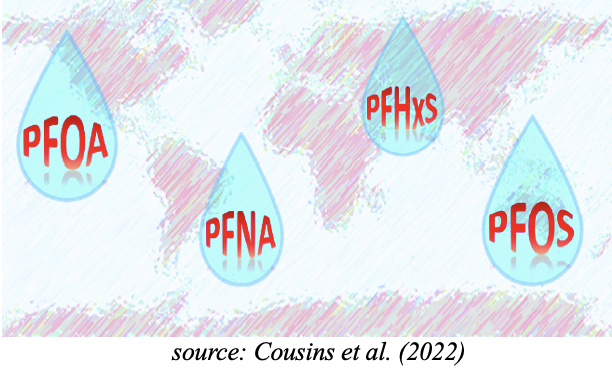
The permanent presence of PFAS in human blood indicates the level of continued exposure of the general population. PFAS are present in drinking water and in food stocks. Hundreds of scientific studies have highlighted the long-term toxicity of PFAS, effecting the liver, kidneys, thyroid and immune system. The ubiquitous presence of PFAS in human blood and other species worldwide highlights the dangers associated with continued manufacturing and use in industrial and consumer products of extremely persistent organic pollutants which are totally anthropogenic in origin, not occurring naturally.
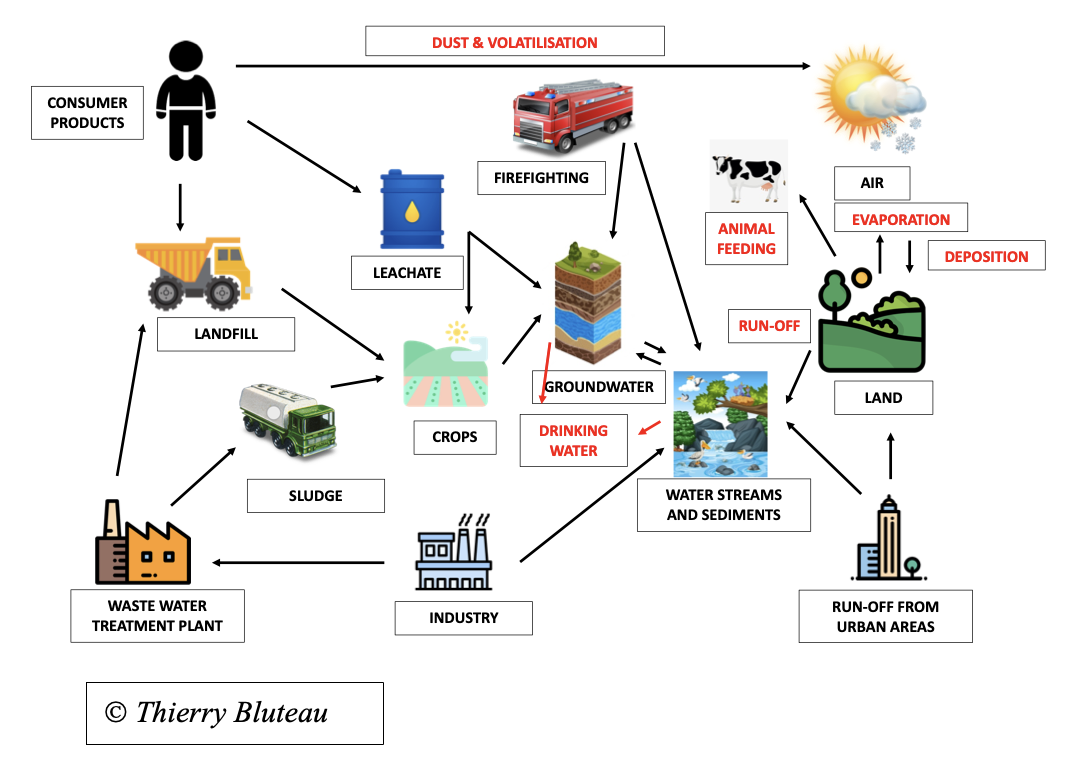
This schema summarizes all possibilities for PFAS to contaminate the environment and living beings including humans.
References
Arp, H.P.M., Hale, S.E., Borchers, U., Valkov, V., Wiegand, L., Zahn, D., Neuwald, I., Nödler, K., and Scheurer, M. (2023) A prioritization framework for PMT/vPvM Substances under REACH for registrants, regulators, researchers and the water sector. German Environment Agency (Umweltbundesamt) Report 22/2023 pp. 1-238.
Bakhshoodeh, R., and Santos, R.M. (2022) Comparative bibliometric trends of microplastics and perfluoroalkyl and polyfluoroalkyl substances: how these hot environmental remediation research topics developed over time. RSC Advances 12, 4973-4987.
Bălan, .A., ChanderMathrani, V., Fengmao Guo, D., and Algazi1, A.M. (2021) Regulating PFAS as a Chemical Class under the California Safer Consumer Products Program. Environ. Health Perspect, 129 (20) February 025001, 1-9.
Blum, A., Balan, S.A., Scheringer, M., Trier, X., Goldenman, G., Cousins, I.T., Diamond, M., Fletcher, T., Higgins, C.P., Lindeman, A.E., Peaslee, G., de Voogt, P., Wang, Z., and Weber, R. (2015) The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs). Environ. Health Perspect. 123 (5) May A107-A11.
Cousins, I.T., Johansson, J.H., Salter, M.E., Sha, B. and Scheringer, M. (2022) Outside the Safe Operating Space of a New Planetary Boundary for Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Technol. 2022, 56, 11172−11179.
European Food Standards Agency (EFSA) (2018) Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA Journal 16(12), 5194.
European Food Standards Agency (EFSA) (2020) Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal 18(9), 6223.
Felizeter, S., McLachlan, M.S., and de Voogt, P. (2014) Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops. J. Agric. Food Chem. 62 (15), 3334-42.
Figuiére, R., Borchert, F., Cousins, I.T., and Ågerstrand, M. (2023) The essential-use concept: a valuable tool to guide decision-making on applications for authorization under REACH? Environmental Sciences Europe 35 (5) 1-12.
Giesy, J.P., and Kannan, K. (2001) Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 35(7) 1339-1342.
Grandjean, P., Andersen, E.W., Budtz-Jørgensen, E., Nielsen, F., Mølbak, K., Weihe, P., and Heilmann, C. (2012) Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307(4), 391-7.
Guy, W.S., Taves, D.R., Brey, W.S. (1976) Fluorocompounds in Human Plasma: Prevalence and Characterization. Biochemistry Involving Carbon-Fluorine Bonds, ACS Symposium. 117-134
Hansen, K.J., Clemen, L.A., Eellefson, M.E., and Johnson, H.O. (2001) Compound-specific quantitative characterization of organic fluorochemicals in biological matrices. Environ. Sci. Technol. 35(4) 766-770.
Hansen K.J., Johnson, H.O., Eldridge, J.S., Butenhoff, J.L., and Dick, L.A. (2002) Quantitative Characterization of Trace Levels of PFOS and PFOA in the Tennessee River. Environ. Sci. Technol. 36(8) 1681-1685.
Kwiatkowski, C.F., Andrews, D.Q., Birnbaum, L.S., Bruton, T.A., DeWitt, J.C., Knappe, D.R.U., Maffini, M.V., Miller, M.F., Pelch, K.E., Reade,A., Soehl, A., Trier, X., Venier, M., Wagner, C.C., Wang, Z., and Blum, A. (2020) Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol., 7, 532−543.
Lerner, S. (2018)] 3M knew about the Dangers of PFOS and PFOA Decades Ago, Internal Documents Show. The Intercept July 18 2018.
Li, J., Sun, J., and Li, P. (2022) Exposure routes, bioaccumulation and toxic effects of per- and polyfluoroalkyl substances (PFASs) on plants: A critical review,. Environ. International 158, 106891.
Moody, C.A., and Field, J.A. (2000) Perfluorinated Surfactants and the Environmental Implications of Their Use in Fire-Fighting Foams. Environ, Sci. Technol. 34(18), 3864-3869.
Mühle, J., Ganesan, A.L. , Miller, B.R, Salameh, P.K., Harth, C.M., Greally, B.R.,, Rigby, M., Porter, L.W., Steele, L.P., Trudinger, C.M.,, Krummel, P.B., O’Doherty, S.,, Fraser, P.J., Simmonds, P.G., Prinn, R.G.,, and Weiss, R.F.. (2010) Perfluorocarbons in the global atmosphere: tetra-fluoromethane, hexafluoroethane, and octafluoropropane. Atmos. Chem. Phys. 10, 5145–5164.
Preston, B.A. (2017) The Judicial Development of the Precautionary Principle. Queenslnad Government Environmental Management of Firefighting Foam Policy Implementation Seminar, Brisbane 21 Febraury 2017. pp. pp.1`-26. < https://lec.nsw.gov.au >.
Rio Convention (1992) UN Conference on Environment and Development, Rio de Janiero, Brazil, 3-14 June 1992.
Roy, M.A., Cousins, I.T., Harriman, H., Scheringer, M., Tickner. J.A., and Wang, Z. (2022) Combined Application of the Essential-Use and Functional Substitution Concepts: Accelerating Safer Alternatives Environ. Sci. Technol. 56, 9842−9846.
Scheringer, M., Trier, X., Cousins, I.T., de Voogt, P., Fletcher, T., Wang, Z., and Webster, T.F. (2014) Helsingør Statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere 114 (2014) 337–339.
Schultz, M.M., Barofsky, D.F., and Field, J.A. (2004) Quantitative Determination of Fluorotelomer Sulfonates in Groundwater by LC MS/MS. Environ. Sci. Technol. 38 (6) 1828-1835.
Sonne, C., Desforges, J.-P., Bossi, R., and Long, M. (2023) Assessment of Exposure to Perfluorinated Industrial Substances and Risk of Immune Suppression in Greenland and its Global Context: a Mixed-Methods Study. Lancet Planetary Health 7 (7), E570-E579, July 2023.
Swanson, L. (2019) Testimony of Lori Swanson Former Minnesota Attorney General before the Committee on Oversight and Reform Subcommittee on Environment United States House of Representatives. September 10, 2019. pp. 1-66.
Taves, D.R. (1966) Normal human serum fluoride concentrations. Nature 211, 192-193.
Taves, D.R. (1968) Evidence that there are Two Forms of Fluoride in Human Serum. Nature 217, 1050-1051.
Wang, W., Rhodes, G., YU, X., and Li, H. (2020) Uptake and accumulation of per- and polyfluoroalkyl substances in plants. Chemosphere 261, 127584.
Wilson, S., Fuglestad, J., Larsen, J.-R., Pawlak, J., and Utne, I. (2017) (AMAP Secretariat). Chemicals of Emerging Arctic Concern. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway. xvi+353pp.
Yoo, H., Washington, J.W., Jenkins, T.M., and Ellington, J.J. (2011) Quantitative determination of perfluorochemicals and fluorotelomer alcohols in plants from biosolid-amended fields using LC/MS/MS and GC/MS. Environ. Sci. Technol. 45(19), 7985-90.
EXPO FIRE – Mexico City
Our sister company 3F MEXICO were present at the professional show EXPO FIRE in Mexico City on the 27th and 28th of August 2024.
The event was a great success and our sales team presented our catalogue of products to more than 250 companies. Some specific products, such as our Fluoro-Free foam FREEGEN, the manikins from RUTH LEE and our automatic systems of extinction KIZENITH for kitchen fires, raised a large interest.


From AFFF to F3 : Fluorotelomers — Part 3
In Part 2 of this series of articles we dealt with Class A foams and the chemistry of legacy Class B AFFF products manufactured using the Simons process of electrochemical fluorination (ECF). In this part current AFFF formulations using fluorotelomers are discussed, which have been manufactured by manufacturers like DuPont, Dynax, Ciba Geigy, Elf Atochem, Daikin, Asahi Glass, Clariant, etc.
Fluoro-telomerisation:
By contrast with the Simons ECF process which produces a mixture of branched and linear isomers with odd and even carbon chain length, fluoro-telomerisation yields almost exclusively linear even-numbered carbon chains (Vyas et al 2007 [3], determined by the starting telogen, i.e. perfluoroethyl iodide (C2F5I) or perfluorobutyl iodide (C4F9I), that is containing carbon chains N, N+2, N+4, N+6, etc.
Telomerisation involves the free radical addition of tetrafluoroethylene (CF2=CF2), the taxogen, to an alkyl iodide, the telogen, such a perfluorobutyl iodide (C4F9I) as shown below. The perfluorinated chain is then terminated with a dimethylene group, -CH2-CH2-, characteristic of end-product fluorotelomers.
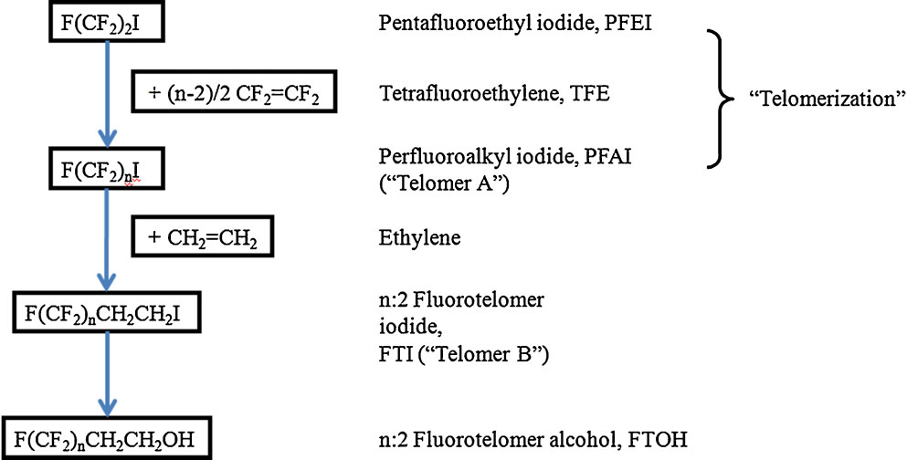
Source: Buck et al (2011)
The starting material is the perfluoroalkyl iodide, whereas the reactive end-product fluorotelomer iodide is used to manufacture a range of end products, e.g., fluorotelomer alcohols, thiols, sulfonic acids and sulfonamides.
Understanding chain length distribution synthesized during telomerisation to yield fluorotelomer iodide is important. Telomerisation produces a homologous series of products with chain lengths consisting of evenly spaced perfluorocarbon units, for example, 4:2, 6:2, 8:2, 10;2, 12:2, 14:2, etc. (N:2 indicates N perfluorinated carbons attached to a non-fluorinated two-carbon unit -(CH2)2-. This is then purified by fractional distillation yielding a fraction containing the shorter chain lengths, i.e., C4-C10 mainly consisting of C6/C8, which has been used mainly for firefighting foams, and longer chain lengths >C8 used for fabric, textile, leather and paper treatments. Other structural variants on the telomer process have occasionally been used by individual manufacturers, including the use of a three carbon spacer, -(CH2)3-, instead of a two carbon unit.
Post the 2010-2015 PFOA Stewardship Program considerable efforts by the fluorochemical industry have managed to reduce the 8:2 fluorotelomer content of the precursor used for firefighting foams to less than 25ppb, as this can act as a precursor for PFOA through breakdown. Early products used to make the fluorosurfactants for formulating firefighting foams were actually a mixture of mainly C6/C8 perfluorinated chain lengths, i.e., 6:2 and 8:2. Modern fluorotelomer foams are now predominantly 6;2 and 4:2 and referred to in the industry as ‘’pure C6’’.
Unfortunately, but predictably, replacement of C6/C8 formulations with ’pure’ C6 fluorotelomers resulted in loss of foam performance which in turn required the use of higher fluorosurfactant concentrations, itself undesirable from an environmental point of view.
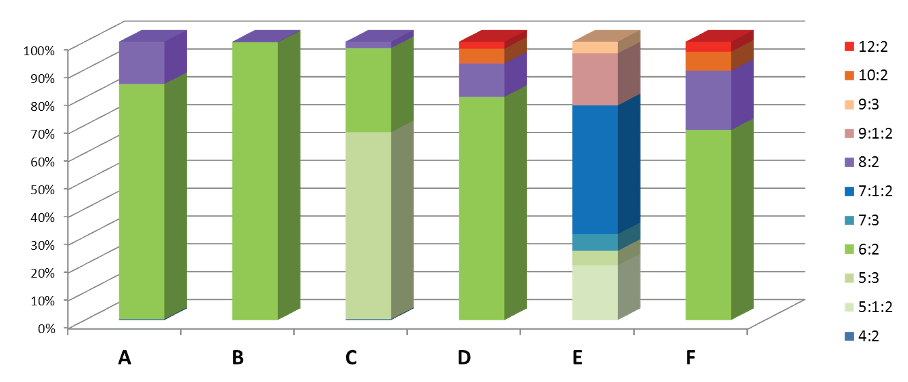
Compositions of six foams ~2005-2010. Data from Backe, Day & Field 2013
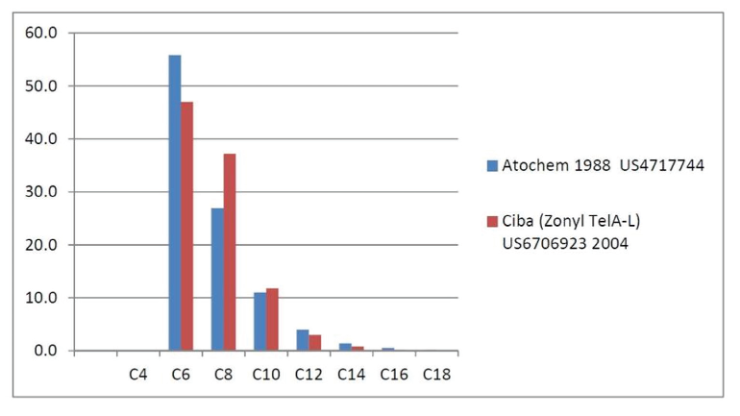
Fluorotelomer Intermediate Homologue Distributions
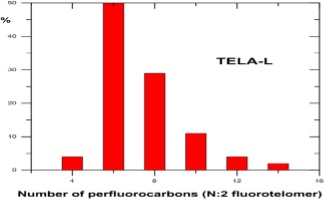
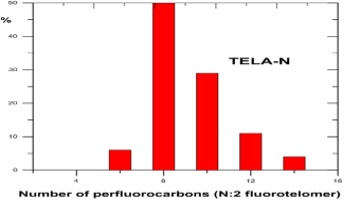
Source : DuPont
Perfluoro compounds are used in firefighting foam to lower surface tension enabling film-formation on many hydrocarbon fuels except those shorter than iso-octane such as hexane or pentane; to provide excellent heat and chemical resistance; to increase hydrocarbon repellency and thus to resist to solvent contamination or ‘f’uel pickup’’; to provide effective vapour suppression.
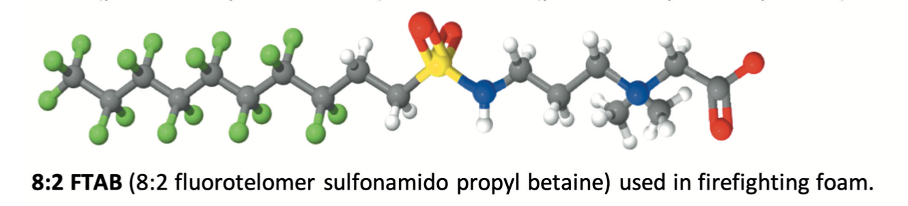
Manufacturers offer a range of perfluorochemicals, most of them being fluorosurfactants. These surfactants are a combination of a hydrophobic and oleophobic perfluorinated tail and a polar head group giving functionality, enabling dispersion or solubilisation of the products in the foam concentrate. One of the most popular and efficient products was probably the C8:2 perfluorinated betaine surfactant together with its C6:2 homologue.
The starting material is the perfluoroalkyl iodide, whereas the reactive end-product fluorotelomer iodide is used to manufacture a range of end products, e.g., fluorotelomer alcohols, thiols, sulfonic acids and sulfonamides.
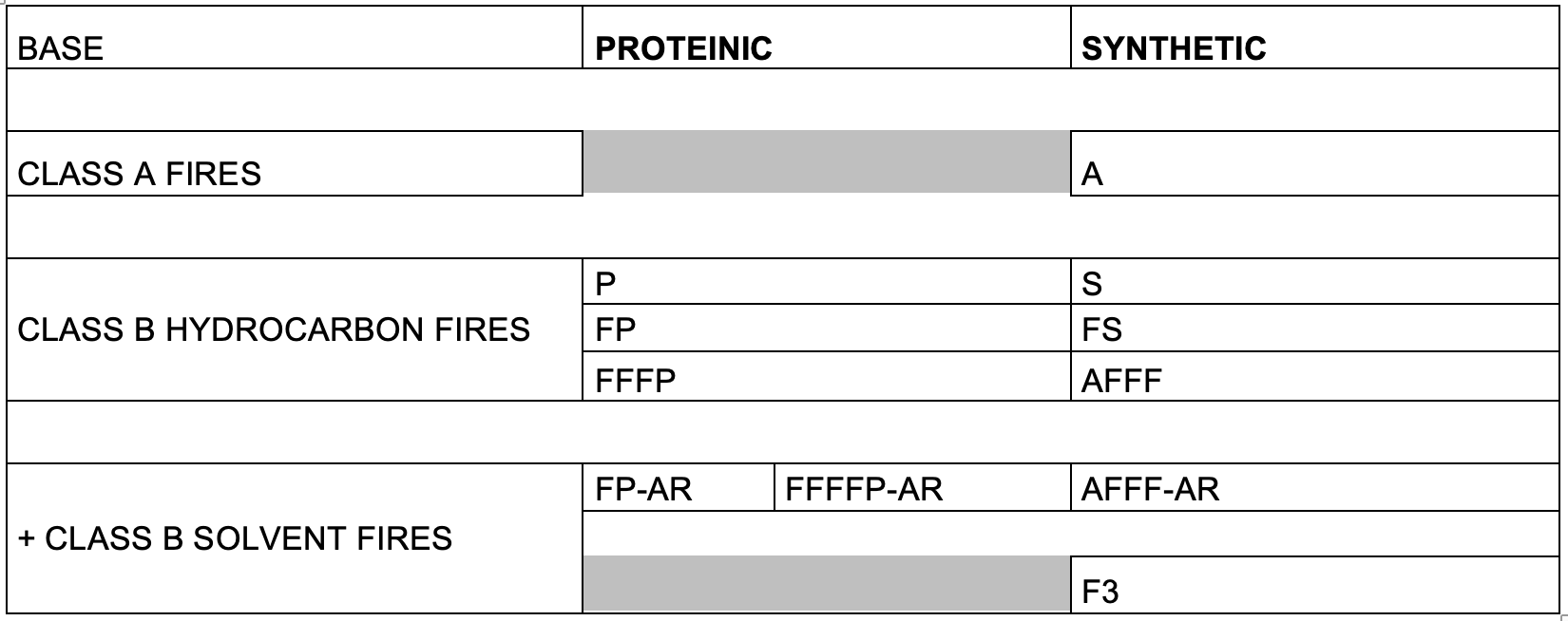
Class B Fluorine-Free Foams (F3) for liquid hydrocarbons and polar solvents
The development of fluorine-free foams (F3) was started in the late 1990s by Ted Schaefer working for 3M Australia. By the early 2000s the first operational fluorine-free firefighting foam, called RF-3 and RF-6 for Rehealing foam 3% and 6% became available. Queensland Fire Service went fluorine-free as early as 2003. Over the next decade or so fluorine-free foam technology greatly improved to the point that today F3 products are available on the market achieving or even in some cases exceeding AFFF performance, whilst offering better value for money. Early developments included Solberg Scandinavian buying the RF patents from 3M as well as Ted Schaefer’s expertise in 2007, as well as the development of F3 by Thierry Bluteau in 2002, then working for Bio-Ex France. In the late 2000s, Gary McDowall (3F Ltd, UK) also developed F3 products. Later on, 3F Ltd offered new F3 solvent-free, i.e., glycol free, thus greatly reducing the BOD-COD problem by around 40-60%. Other major manufacturers followed suite and today F3 firefighting foams are widely available on the market, with many major organisations in civilian and military aviation, oil and gas and petrochemical industries, as well as large municipal fire departments transitioning from fluorine-containing AFFF to fluorine-free F3 foams.
The transition has taken nearly 10-15 years, mainly due to built-in conservatism in many fire departments, but also because of the costs involved which include modifying or cleaning existing equipment, as well as the proper and expensive disposal of existing legacy stocks of AFFF. Another driving force, especially in the US, has been the increasing financial and legal exposure of continuing to use products which give rise to persistent and widespread environmental contamination.
The environmentally sustainable destruction of legacy AFFF stocks, often involving huge volumes of concentrate running to millions of litres, requires destruction methods that are highly efficient (> 99.999% DRE), capable of handling solid and liquid charges, do not further contaminate the environment, and are financially feasible. Methods that are currently available will be discussed in a further article.
Apart from using fluorocompounds for their exceptional physicochemical properties, firefighting foam contains a range of other chemicals which are necessary to achieve the extinction.
The main components found in firefighting foam with or without fluorosurfactants or fluoropolymers include the following:
Foaming agents:
(a)Some fluorosurfactants like PFOS and PFHxS and their functionalised derivatives, or fluorotelomer compounds such as 1157 (perfluoroalkyl betaine) or 1183 (perfluoroalkyl aminoxide) have been used occasionally to boost foam volume in AFFF foams.
(b)r A large range of hydrocarbon surfactants are widely used by manufacturers in all types of synthetic foams: AFFF, AFFF-AR, High Expansion, Class A and F3.
Synthetic surfactants: are made from hydrocarbon chain precursors (e.g., CH3(CH2)n-produced by the petrochemical industry from mineral oil and/or animal and plant fatty acids, which are then functionalized with a polar head-group to obtain the desired surfactant property, for example, octyl sulfonate, CH3(CH2)7SO3-, or dodecyl sulfate, CH3(CH2)11SO4-.
(c) Protein polymer: obtained from the hydrolysis of slaughterhouse waste ‘‘horn and hoof’’, this old-fashioned and polluting process consists of heating the raw material in highly alkaline media. The keratin is degraded into small protein fragments, followed by neutralisation and stabilisation. The concentrated end-product can be contaminated with haemoglobin from residual blood giving it a very characteristic dark brown colour. Under operational conditions protein foams are characteristically dark brown in colour with a highly distinctive smell especially when applied to a fire.
Foam stabilisers: most of them are glycol ethers. The most used are butyl glycol, butyl carbitol and hexylene glycol, and more recently ethyl or butyl propylene glycols. We can find too lauryl alcohol.
Anti-freeze agents: monoethylene glycol, (CH2OH)2, and mono-propylene glycol, CH2(CH2OH)2, are widely used, but manufacturers also use sodium chloride, urea, etc, in some formulations.
The glycols and glycol ethers present in foam formulations are at relatively high concentrations – typically 10-20% – and are the major contributors to the BOD/COD value.
Other additives: in this category formulators use preservatives, anti-corrosion products, buffers to stabilise foam pH, and chelating agents for ions that would degrade foam performance, all at levels below 1%.
Natural polymers: carbohydrate xanthan gum is a very common natural polymer used to give alcohol-resistance to the foam. Applied to a burning fuel surface, the polymer precipitates and chars forming a barrier which resists and prevents contamination of the foam blanket by fuel – ‘’fuel pickup’’. Other polymers and gums are also used, such as celluloses, alginates, guar, locust bean, or carrageenan.
The tables below summarise the main properties of principal ingredients used in formulations.
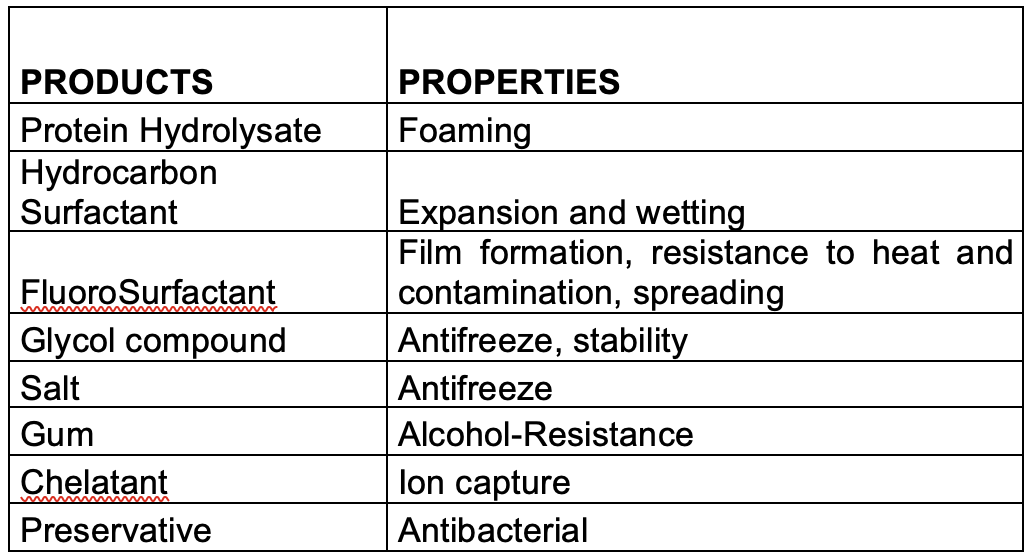
Currently, there are at least 12 different types or foam on the market, some of which have declined in the volume used over recent years.
Different users have different hazards associated with specific risks. In selecting the correct foam, it is important to do a suitable and sufficient assessment of these specific risks, ensuring that the foam chosen is ‘fit-for-purpose’, and then go through the following steps during procurement and operational use:
(a) list the equipment: whether this is fixed or mobile, i.e., tank farm, monitors or fire appliances;
(b) check the correct induction rate, e.g., 1%, 3% or 6%, for use;
(c) ensure that the application rate is suitable;
(d) determine the length of time that the foam should be applied, and the foam blanket stability and when re-application is necessary;
(e) determine the availability of possible support from external sources, i.e,. reinforcement;
(f) be aware of the manufacturer’s warranty and specified operating conditions for the foam;
(g) consider local environmental regulations – both current and any likely
3F is a responsible manufacturer and will be pleased to assist any of its customers in the assessment of risks and selection of an appropriate foam and associated equipment.
To be continued in Part 4.
References
Benskin J.P., De Silva A.O., Martin J.W. (2010) Isomer Profiling of Perfluorinated Substances as a Tool for Source Tracking: A Review of Early Findings and Future Applications. Rev. Environ. Contam. Toxicol.:111-160.
Buck, R.C., Franklin, J., Berger, U., Conder, J.M., Cousins, I.T., de Voogt, P., Jensen, A.A., Kannan, K., Mabury, S.A., and van Leeuwen, S.P.J. (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 7(4), 513-541.
D’Agostino, L.A, and Mabury, S.A. (2014) Identification of Novel Fluorinated Surfactants in Aqueous Film Forming Foams and Commercial Surfactant Concentrates. Environ. Sci. Technol. 48(1):121-9.
Moe, M.K., Huber, S., Svensen, J., Hagenaars, A,. Pabon, M., Trümper, M., Berger, U., Knapen, D., and Herzke, D. (2012) The structure of the fire fighting foam surfactant Forafac®1157 and its biological and photolytic transformation products. Chemosphere 89(7), 869-875.
Naile, J., Garrison, A.W., Avants, J.K., and Washington, J.W. Isomers/Enatiomers of Perfluorcarboxylic Acids: Method Development and Detection in Environmental Samples. Chemosphere 44, 1722-1728.
Sasaki, T., Egami, A., Yajima, T., Uekusa, H., and Sato, H. (2018) Unusual Molecular and Supramolecular Strcuturs of Chiral Low Molecular Weight Organogelators with Long Perfluoroalkyl Chains. Crystal Growth and Design 18(7) 4200-4205.
From AFFF to F3 : Chemistry – Part 2
In this part we deal with the chemistry involved in formulating Class A foams for carbonaceous fuels, and legacy Class B AFFF foams based on PFOS chemistry for liquid hydrocarbon and polar solvent fires.
In Part 1 of this series of articles we saw that AFFF firefighting foams contain various perfluoroalkyl substances (PFAS); however, firefighting foam is only one of many applications.
PFAS have been used for decades in more than 200 other industrial and domestic applications, such as food packaging, leather and textile treatment, carpet and clothing anti-stain protection, detergents, water-proofing and oil-proofing, paints and varnishes, printing inks, chromium plating, outdoor and protective clothing (PPE) for the emergency services and military. These perfluorinated substances are widely used as they offer a combination of unique properties, including the ability to repel water (hydrophobicity), the ability to repel oils (oleophobicity), the ability to reduce the surface tension of aqueous solutions to less than 20 dyne/cm and with it acting as detergents, emulsifiers, wetting agents, and dispersants.
The OECD (2021) has recently clarified the definition of what constitutes a PFAS, whilst acknowledging that given by Buck et al (2011), as follows:
“PFASs are defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS.”
More than 800 products currently available in the marketplace have been identified, but the true list of PFAS used in commerce and industry is likely to be 10,000 or more; the UN Stockholm Convention has listed 4,700 substances related to PFOA alone. PFAS started to be manufactured in large quantities in the early 50’s. All of them are anthropogenic created by humans using chemical synthesis. They do not exist naturally. Their extremely stable and chemically resistant perfluorinated end-products of breakdown in the environment have long been identified as ‘forever chemicals’, for example by scientists and journalists such as Rebecca Renner [“Growing Concern Over Perfluorinated Chemicals” (2001) Environ. Sci. Technol. 35(7) 154A-160A; “The long and the short of perfluorinated replacements” (2006) Environ. Sci. Technol. 40(1) 12-13] or Sharon Lerner writing in the Intercept [“Toxic Chemicals Discovered in Hundreds of Products” Sharon Lerner (The Intercept December 2020)].
It must be stressed that although still commonly and inaccurately referred to as ‘emerging contaminants’, PFAS have truly emerged as contaminants of concern for at least 10 years and should no longer be described as ‘emerging’. On the other hand, the technology of how to deal with PFAS waste is currently still emerging and developing.
Firefighting foams are classified either as Class A suitable for carbonaceous fuels such as wood, paper or vegetation, acting as wetting agents improving the penetration of water into deep seated fires and do not contain fluorosurfactants, only hydrocarbon surfactants; or, on the other hand, Class B foams are specifically formulated for liquid hydrocarbons such as gasoline and polar solvents such as ethanol. Modern Class B foams may either contain fluorsurfactants and be capable of film-formation at the air-fuel interface (AFFF), or completely fluorine-free, F3 foams, specially formulated containing only hydrocarbon surfactants. Interestingly Class B fluorine-free foams (F3) can be used effectively for both Class A and Class B fires unlike AFFF,
Class A foams for carbonaceous fuels
Class A firefighting foams are used extensively worldwide, especially in Australia, America and Southern Europe, for incidents involving carbonaceous fuels, e.g., structural house fires, plastic and tyre waste, as well as grassland and wildland or bush fires. Ted Schaefer then working for
3M Australia in the late 1980s developed one of the first effective Class A foams, “3M Fire-Brake BFFF”, recognised in 2001 by the Australian Academy of Technological Sciences and Engineering as one of the top 100 Australian inventions of the 20th century.
Class A foams behave very differently to fluorosurfactant-containing AFFFs, as they are specifically formulated to penetrate carbonaceous fuel effectively, such as compacted vegetation, paper or wood, using specialised hydrocarbon surfactants, not unrelated to kitchen washing-up liquid. Fluorosurfactant AFFFs, designed for surface application to liquid hydrocarbon or polar solvent fires, are nowhere nearly as efficient at penetrating such deep-seated fires and claims by some in the industry that their AFFF products can be used as dual Class A / Class B foams is frankly misleading.


Mister H: Penetration by Class A Mister F: Failure to penetrate by Class B AFFF
(Bluteau 2007)
Class B AFFF foams for liquid hydrocarbons and polar solvents
The first report of an aqueous film-forming foam (AFFF), called LightWater®, by R.L. Tuve et al of the Naval Research Laboratory and the 3M Company March 1964 of a foam capable of vapour suppression and film forming on the surface with low flash point flammable fuels such as gasoline, showed that it was 1200% more effective than standard protein foams under identical conditions.
The compounds tested in foam formulations were in the general class of perfluorosulfonic acid derivatives, some being quaternary salts, others being alcohols, esters, anionic salts of substituted sulfonamido carboxylic acids, etc. All of these water soluble, high molecular weight fluorocarbons shown dramatic surface tension depression of water to below 20 dynes/cm. In general they are insensitive to electrolytes and show surface activity when dissolved in organic solvents.
The first Patent for an AFFF was granted to Richard Tuve and Edwin Jablonski in June 1966 [1], representing a new era in firefighting foams which was to last for the next 30-40 years until the 3M Company Minnesota withdrew from PFOS-based chemistry altogether in May 2000.
Information from the patent literature gives a fascinating insight into the derivatives used in these early AFFFs. Derivatives of perfluorooctane sulfonamide (PFOSA) and perfluorooctane carboxylic acid (PFOA) were used. As reported in the 1966 patent these early formulations include the quaternary ammonium salts of PFOS and PFOA amido derivatives:
C8F17-SO2NH2-(CH3)3N(CH3)3+I-
C7F15-CONH-(CH3)3N(CH3)3+I-
an amphoteric amino betaine derivative of PFOA
C7F15-CONH-(CH2)3–N+(CH3)2-CH2-CH2-COO–
and the potassium salt of a PFOS sulphonamide derivative
C8F17SO2N(C2H5)-CH2COOK
The potassium salt of PFOS in the form of surfactant FC-95 was also used in early foams.
Interestingly it was some 50 years later that Barzen-Hanson et al in 2017 [2] from Jennifer Field’s group at Oregon State University identified a vast range of other derivatives, or their breakdown products, involving 40 different classes in legacy AFFFs.
Electrochemical Fluorination (ECF) – the Simons Process
The 3M Company announced in May 2000 that it was phasing out fluorosurfactant production based on PFOS chemistry and withdrawing entirely from the fluorinated AFFF firefighting foam market marking an end to the availability of Light Water™ and Light Water™ ATC™ formulations (3M Company (2000)). Other products using PFOS included ScotchGuard™ stain and water repellent treatments. Production of PFOS by the 3M Company is thought to have ceased entirely around 2002, being replaced by the shorter chain compound PFBS, although PFOS and PFHxS production is thought to have continued in China and India.
Until 2000 PFOS had been manufactured using the Simons electrochemical fluorination (ECF) process (3M Company, 1999; Ignat’ev et al , 2009; Sartori and Ignat’ev, 1998). This process involves replacing the hydrogen atoms of octyl sulfonate using hydrogen fluoride electrolytically in order to generate perfluorooctane sulfonyl fluoride, PFOSF.
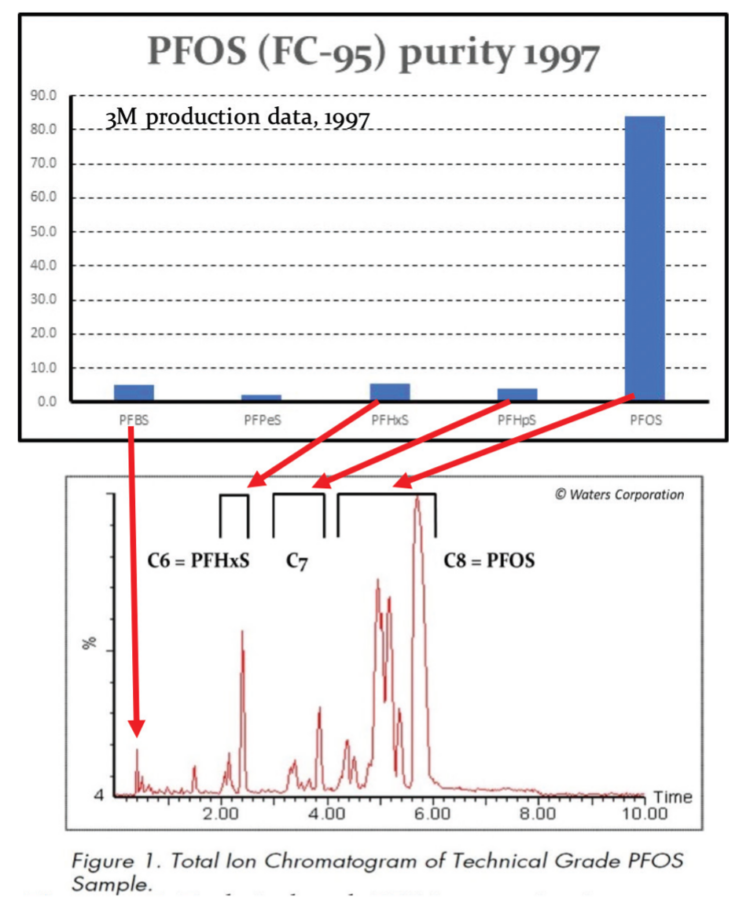
C8H17SO3H + HF ==>> C8F17(C=O)F
PFOSF is highly reactive acyl fluoride and is the starting material for preparing PFOS derivataives such as the sulfonamide PFOSA or N-ethyl-PFOSA, for example:
C8F17(C=O)F + C2H5NH2 ==>> C8F17(C=O)-NH-C2H5
PFOSF production using electro-chemical fluorination (ECF) was, and remains, an inherently ‘dirty’ process resulting in a wide range of structural isomers, both straight chain and branched with CF-CF3 and C-(CF3)2 side chains, as well as odd and even chain length homologues such as C4 PFBS, C6 PFHxS and C7 PFHpS. As a result, technical grade PFOS was always and continues to be contaminated with a significant percentage of PFHxS. In addition, the perfluoroalkyl chains of both PFOS and PFHxS can form left- or right-handed helices resulting in pseudo-racemates that have been detected in human sera (Wang et al, 2011; Naile et al , 2016; Sasaki et al , 2018). Quoting from the ECHA (13 June 2019) PFHxS restriction proposal:
“…Sources indicate that when manufacturing perfluorinated compounds, a mixture of compounds of varying chain- length is usually formed, with typical amounts of PFHxS formed when manufacturing PFOS being between 4 and 14% ( from (BiPRO, 2018) citing (Ren, 2016). These numbers are supported by measurements of PFHxS in commercial PFOS-products, namely 3.5%–9.8% in 3M’s FC-95 (from (BiPRO, 2018) citing 3M (2015) and 11.2 % – 14.2% in three products from China (Jiang et al, 2015). BiPRO also note, however, that the amount of the C6-component may be reducedby purification at different stages of the production line….”
The significance of the relatively high levels of the C6 homologue perfluorohexane sulfonic acid, PFHxS, in these AFFF formulations is that PFHxS is more toxic and bioaccumulative than PFOS, has a longer biological half-life in humans, and has also been list in the Annexes of the UN Stockholm Convention for restriction. Unfortunately, some manufacturers especially in Asia have used PFHxS as a ‘regrettable substitution’ for PFOS.
The use of ECF to produce perfluorinated sulfonic and carboxylic acids, such as PFOS and PFOA and their derivatives, has been summarised by Buck et al [2011], as shown below.
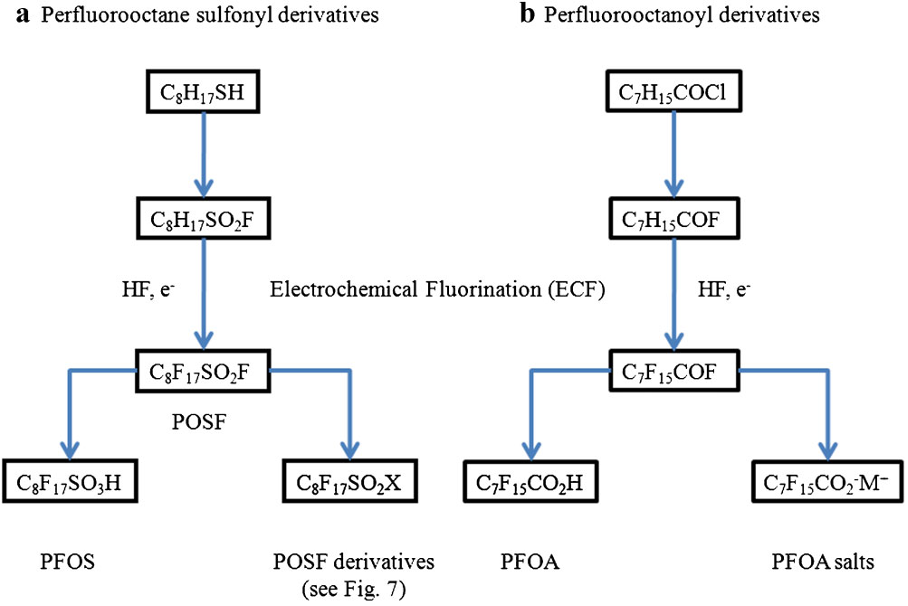
source: Buck et al (2011)
To be continued as Part3.
References
Barzen-Hanson, K.A., Roberts, S.C., Choyke, S., Oetjen, K., McAlees, A., Riddell, N., McCrindle, R., Ferguson, P.L., Higgins, C.P., and Field, J.A.. (2017) “Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater” Environ, Sci. Technol. 51, 2047-2057.
Benskin J.P., De Silva A.O., Martin J.W. (2010) Isomer Profiling of Perfluorinated Substances as a Tool for Source Tracking: A Review of Early Findings and Future Applications. Rev. Environ. Contam. Toxicol.:111-160.
Buck, R.C., Franklin, J., Berger, U., Conder, J.M., Cousins, I.T., de Voogt, P., Jensen, A.A., Kannan, K., Mabury, S.A., and van Leeuwen, S.P.J. (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 7(4), 513-541.
Ignat’ev, N.V., Willner, W., and Sartori, P. (2009) Electrochemical fluorination (Simons process) – A powerful tool for the preparation of new conducting salts, ionic liquids and strong Brǿnsted acids. J. Fluorine Chem. 130(12), 1183-1191.
Naile, J., Garrison, A.W., Avants, J.K., and Washington, J.W. Isomers/Enatiomers of Perfluorcarboxylic Acids: Method Development and Detection in Environmental Samples. Chemosphere 44, 1722-1728.
OECD (2021), Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances:
Recommendations and Practical Guidance, OECD Series on Risk Management, No. 61, OECD
Publishing, Paris.
Sartori, P. and Ignat’ev, N.V. (1988) The actual state of our knowledge about mechanism of electrochemical fluorination in anhydrous hydrogen fluoride. J. Fluorine Chem. 87(2(, 157-162.
Sasaki, T., Egami, A., Yajima, T., Uekusa, H., and Sato, H. (2018) Unusual Molecular and Supramolecular Structures of Chiral Low Molecular Weight Organogelators with Long Perfluoroalkyl Chains. Crystal Growth and Design 18(7) 4200-4205.
Tuve, R.L. amd Jablonksi, E.J. (1966) US 3,258,423 Patent June 28, 1966 “Method Of Extinguishing Liquid Hydrocarbon Fires”, assignors to the United States of America as represented by the Secretary of the Navy. Filed Sept. 4, 1963, Ser. No. 306,665.
Vyas, S.M., Kania-Korwel, I., Lehmler, H.J. (2007) Differences in isomer composition of perfluorooctanoylsulfonyl (PFOS) derivatives. J. Environ. Sci. Health and Toxic Hazard Substance Environ. Eng. 42, 249-255.
Wang, Y., Beeson, S., Benskin, J.P., De Silva, A.O., Genuis, S.J., and Martin J.W. (2011) Enantiomer Fractions of Chiral Perfluoroctanesulfonate (PFOS) in Human Sera. Environ. Sci. Technol. 45(20) 8907-8914.
From AFFF to F3 : History – Part 1
Modern chemistry has created many hundreds of thousands of chemical compounds, which we encounter as part of our daily lives. Indeed, it would be very hard to spend a day without being in contact with a class of molecules – perfluoroalkyl substances or PFAS – which have been commercially exploited since the end of WWII over the last 75 years.
Even if we could qualify chemistry as a miracle of science, it is worth knowing that chemistry has been approached by the Egyptian 3000 years BC, and was later on studied by the Ancient Greeks which described the combination of the 5 elements: earth, air, fire, water and ether. This theory was largely accepted for more than 1000 years.
The basis of the modern chemistry, as we now understand it, was established over the last three hundred years. The nature of the atom, the identification of atomic compounds, the first modern synthesis was achieved.
In 1906, Frédéric Henri Moissan (1852-1907), a French chemist working in Paris at the École Supérieure de Pharmacie, isolated for the first-time elemental fluorine gas, F2, a discovery for which he was awarded the Nobel Prize in Chemistry in 1906. Hydrogen fluoride obtained from fluorspar had been identified by the renowned Swedish chemist Karl Wilhelm Scheele some years earlier.
The post war years after WWI In the mid 1930s, were the heyday the German chemical industry, especially as regards new synthetic dyes. Fluorine chemistry started to have a commercial role – a red dye Naphthol AS, used as the official red colour the Nazi flag, and Indanthrene Blue used as a component of ‘Flieger Grau’ or Pilot Grey, the blue-grey colour of Luftwaffe uniforms, both contained a fluorinated methyl group, CF3, which helped prevent fading. Carothers working for DuPont produced the first industrial wholly synthetic fabric, the polymer Nylon. From this point chemists have never stopped to inventing new compounds!
When we think about chemicals, it is important to realise that many, if not most of the synthetic compounds now commercially available are produced by the petrochemical industry. Since the invention of the internal combustion engine, petrol (gasoline) and petroleum products have been become increasingly important around in for many human activities but are associated with a high fire risk.
The nature of this risk highlighted the necessity of addressing these often-catastrophic fires. In the early 40’s, protein foam made from ‘horn and hoof’, a slaughterhouse waste, was developed to control Class B hydrocarbon fires, e.g., those involving oil, gasoline, aircraft fuel and solvents.
In 1949, the 3M Company Minnesota industrialized the Simons electrochemical fluorination (ECF) process for making perfluoro-compounds (PFC) such as perfluorinated amines, carboxylic and sulphonic acids in which the hydrogens of the alkyl carbon chain had been totally replace by fluorine. Joseph Simons had discovered the ECF process whilst working at Pennsylvania State College in the 1930s but was unable to publish his work until after WWII because fluorine chemistry was essential for uranium purification as part of the Manhattan Project.
In 1953 the structure of Scotchgard was accidentally discovered by Patsy Sherman and Sam Smith working for the 3M Company whilst working on a rubber for jet fuel lines. Three years later in 1956, the 3M Company launched Scotchguard on the market. This fabric, textile and leather treatment is based on a PFOS-derivative containing N-ethyl-PFOSA and gives water, oil, and other liquids and stain-protection to the treated fibre.
Interestingly, N-ethyl-PFOSA known as Sulfluramid, was originally developed to kill ants, cockroaches and termites, and is still used to this day as the insecticide sulfluramid against leaf-cutting ants in Brazil. The lithium salt of PFOS was developed to kill wasps and hornets but is highly toxic to honey bees.
PFOS, perfluoro-octane sulphonic acid, and its derivatives subsequently become crucial for the development of Class B aqueous film-forming foams (AFFFs) effective against liquid hydrocarbon and solvent fires.
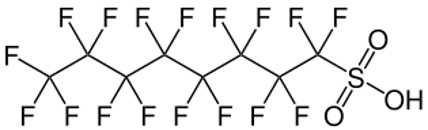
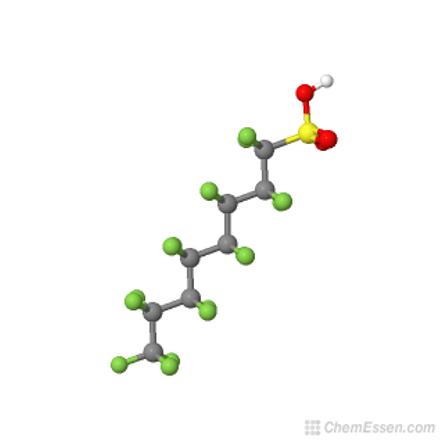
Figure 1. The structure of PFOS, perfluoro-octane sulphonic acid.
During the 1960s the US Department of the Navy Naval Research Laboratory in collaboration with the 3M Company began developing PFOS-based firefighting foams. A patent for AFFF firefighting foam was awarded in June 1996 for extinguishing liquid hydrocarbon fires.
In the late 60’s, a series of major fuel fires happen on board US Navy ships causing extensive loss of life and damage:
(i) 1966: USS Oriskany – fire kills 44 sailors.
(ii) 1968: USS Forrestal – whilst on active service in the Gulf of Tonkin during the Vietnam War, malfunction and accidental firing of a fighter Zuni rocket on the flight deck of this super-carrier led to a catastrophic aviation fuel fire claiming the lives of 134 crew, injuring many more, destroying nearly 50 aircraft, doing $72 million worth of damage, and leaving the vessel unfit for active service.

(iii) 1969: USS Enterprise – a shipboard fire kills 28 sailors.
These major fires prompt the US Department of the Navy to mandate the use of the recently developed AFFF firefighting foam, which the 3M Company was manufacturing for the US military.
Perfluorocompounds had been successfully used to create AFFF, and 3M’s PFOS-based LightWater® and alcohol-resistant ATC® brands became the staple for liquid hydrocarbon fuel fires from the 1970s until May 2000 when the Company announced that it was phasing out PFOS-based chemistry on environmental grounds. AFFF had indeed conquered the world of firefighting and was seen for decades as the ultimate answer to extinguishing large hydrocarbon (oil and gasoline) fires both for military and civilian use especially by the aviation and petrochemical industries.
In the 70’s, an alternative technology was developed based on the telomerization process. This technology provided an alternative to the ECF process and introduced a new class perfluorochemicals on the market. Whereas the ECF process produced mainly PFOS contaminated with odd and even numbered homologues of PFOS such as PFHxS (~5-8 % w/w), perfluorohexane sulphonic acid, as well as branched chain isomers, telomerisation produced only even number linear alkyl carbon chains. The characteristic of fluorotelomer derivates is a perfluoroalkyl moiety linked by a dimethylene group -CH2-CH2- to a functional group which could be negatively (anionic), positively (cationic) or both negatively and positively charged (amphoteric).
Most modern fluorotelomer-based AFFF firefighting foams, known as ‘pure C6 foams’, are based on derivatives of 6:2 fluororelomer sulphonic acid (6:2FTS), or a thioether analogue, containing a C6 perfluoroakyl chain linked through -(CH2)2- to a charged functional group. 6:2FTS contains a C8 chain and its structure is shown below. Its similarity to PFOS is clear but the CH2 groups cause it to behave very differently in terms of its PBT profile. All perfluoroalkyl moieties or their breakdown products are extremely environmentally persistent (vP), but with differing bioaccumulation or toxic potential.
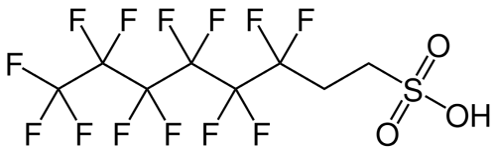
Figure 2. Structure of 6:2FTS
Early fluorotelomer foams, however, contained both 6:2FTS and often substantial amounts of 8:2FTS derivatives. This was a problem as the 8:2FTS could be degraded to a stable end product perfluoroctanoic acid or PFOA which had substantial toxicity. This problem has now been essentially resolved as a result of the industry PFOA Stewardship Program 2010-2015, with residual PFOA or its precursors reduced to less than 25 parts perbillion (ppb).
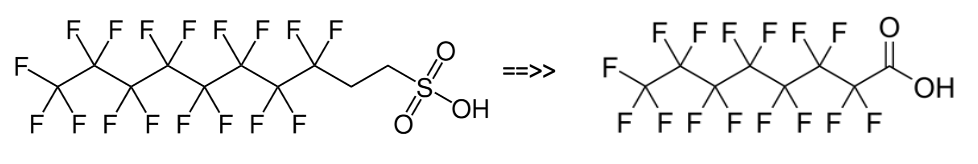
Figure 3. 8:2FTS breakdown to PFOA
From the 1970s to the late 1990s, many manufacturers of firefighting foam appeared in the market, developing and offering a wide range of different foams for end-users. These included Class B AFFF, AFFF-AR (alcohol resistant), film-forming-protein (FFFP) and fluoro-protein (FP) foams. Class A foams specifically intended for solid carbonaceous fires such as structural building or wildland (bush) fires were also developed in this period.
On 16 May 2000 the 3M Company abruptly announced the phasing-out of its activity in PFOS-based chemistry for producing fluorochemicals, affecting not only firefighting foams but also a wide range of domestic and commercial products. This announcement was justified on company responsibility for environment, as it was confirmed that the C8 perfluoroalkyl substances (PFAS) made using ECF technology posed a threat to the environment, with pollution that had spread worldwide affecting a wide range of environmental compartments as well as biota including man.
Over the next 2-3 years, the company had stopped all activities involving PFOS-based chemistry, with a total withdrawal from the firefighting foam market, replacing it with mitigated success by a shorter chain PFBS-based (perfluorobutyl sulphonate) chemistry. However, some production of PFOS and PFHxS derivatives using the ECF process did continue in both China and India.
With 3M’s phase-out of PFOS-chemistry and withdrawal from the firefighting foam market, other major manufacturers of PFAS fluorochemicals and firefighting foam stressed that they considered fluorotelomer chemistry was ’safe’ and indeed environmentally friendly as it had nothing to do with ECF chemistry and products could not contain either PFOS or PFOA. The firefighting foam market transitioned to AFFF products based on fluorotelomers over the next few years 2000-2010.
From 2002 onwards a lively and sometimes acrimonious debate took place between manufacturers of PFAS and AFFF – under the auspices of a trade association, the Fire Fighting Foam Coalition (FFFC), funded mainly by the fluorochemical industry – and independent manufacturers especially of nascent fluorine-free foams (F3), regulators and scientific experts from academia. This discussion gave rise to a series of international seminars, conferences as well as hundreds of publications in the peer-reviewed literature about the environmental consequences of substituting fluorotelomers for PFOS-based products. At this time the main international forum for discussing developments in firefighting foam technology turned out to be the Reebok series of foam conferences, held in Manchester and Bolton in the UK, 2002, 2004, 2007, 2009 and 2013.
Starting as early as 2002 a number of the smaller independent foam manufacturers started to offer first generation experimental Class B liquid hydrocarbon fluorine-free foams (F3) as more environmentally sustainable alternatives to PFAS -containing foams. During the following 10 years the debate raged on driven by published scientific studies which concluded that telomer chemistry posed a threat to the environment. Early telomer formulation were mixtures of 6:2 and 8:2 derivatives. The 8:2 material was shown to be a potential precursor for the generation of environmentally extremely persistent PFOA (perfluorooctanoic acid or C8) through breakdown, subsequently associated with long-term health effects. At the time this was vigorously contested by representatives advocating the fluorochemical industry attending Reebok conferences.
However, as a result of pressure from the US EPA many large feedstock manufacturers adopted the PFOA Stewardship Program 2010-2015 aimed at reducing the use of PFOA or its precursors. Improvements in purifying the fluorotelomer derivatives resulted in a reduction of PFOA related material to less than 25 ppb providing so-called ‘pure C6’ fluorotelomer derivatives. The Stewardship Program led to a change in foam formulations formerly containing C6/C8 fluorotelomers to a so-called ‘drop in’ replacement containing predominantly C6 fluorotelomer.
Unfortunately, this change was not as simple as it was supposed to be and foam manufacturers had to reformulate and increase the total fluorochemical content to achieve a similar performance compared with previous C6/C8 formulations. Unfortunately, end-users were not even made aware of this change!
Around the same time, scientific studies accumulated evidence that even hyper-pure C6 was NOT a suitable alternative, but a ‘regrettable substitution’ and the debate went to another level. 2015 marked a sea-change in the PFAS debate. The toxicity of PFOA was no longer denied by the fluorochemical industry or regulators and the issue was brought to the attention of the public by scientific journalists.
A series of public statements signed by scientists worldwide – the Helsingǿr Statement 2014, the Madrid Statement 2014 and the Zürich Statement 2018 – raised concerns about the continuing use of PFAS and as major planetary pollutants and their long-term impact on the environment. A major publication in 2020 raised the issue that all PFAS should be treated as a chemical class because of their common environmental problems rather than individual chemicals.
The United Nations Stockholm Convention and their Persistent Organic Pollutants Review Committee (POPRC) has added PFOS, PFHxS and PFOA to the appropriate Annexes banning or restricting use (2018-2022).
Countries such as Germany and Norway, or individual states such Queensland in Australia, have been at the forefront in regulating the use of PFAS, especially for highly dispersive use such as firefighting foams.
Some countries are not waiting for UN decisions to regulate the use of PFAS. In Europe, PFOS has been prohibited since 2011 and PFOA since 2018; current discussions aim to stop the use of all PFAS with C4 to C20 carbons completely with a deadline of 2025, in anticipation of these restrictions many industries are moving to fluorine-free technology. In the USA, change is being driven mainly by the cost of litigation with thousands of cases against the fluorochemical industry including foam manufacturers in the pipeline.
To be continued in Part 2
