From AFFF to F3 : Fluorotelomers — Part 3
In Part 2 of this series of articles we dealt with Class A foams and the chemistry of legacy Class B AFFF products manufactured using the Simons process of electrochemical fluorination (ECF). In this part current AFFF formulations using fluorotelomers are discussed, which have been manufactured by manufacturers like DuPont, Dynax, Ciba Geigy, Elf Atochem, Daikin, Asahi Glass, Clariant, etc.
Fluoro-telomerisation:
By contrast with the Simons ECF process which produces a mixture of branched and linear isomers with odd and even carbon chain length, fluoro-telomerisation yields almost exclusively linear even-numbered carbon chains (Vyas et al 2007 [3], determined by the starting telogen, i.e. perfluoroethyl iodide (C2F5I) or perfluorobutyl iodide (C4F9I), that is containing carbon chains N, N+2, N+4, N+6, etc.
Telomerisation involves the free radical addition of tetrafluoroethylene (CF2=CF2), the taxogen, to an alkyl iodide, the telogen, such a perfluorobutyl iodide (C4F9I) as shown below. The perfluorinated chain is then terminated with a dimethylene group, -CH2-CH2-, characteristic of end-product fluorotelomers.
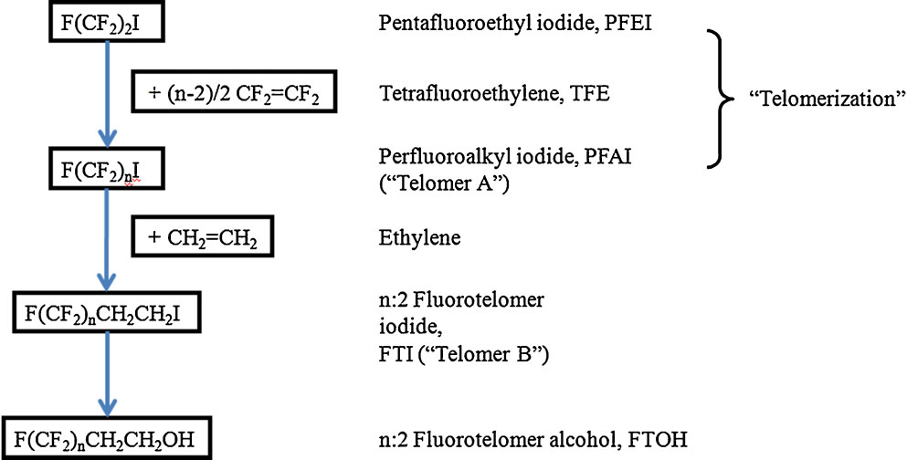
Source: Buck et al (2011)
The starting material is the perfluoroalkyl iodide, whereas the reactive end-product fluorotelomer iodide is used to manufacture a range of end products, e.g., fluorotelomer alcohols, thiols, sulfonic acids and sulfonamides.
Understanding chain length distribution synthesized during telomerisation to yield fluorotelomer iodide is important. Telomerisation produces a homologous series of products with chain lengths consisting of evenly spaced perfluorocarbon units, for example, 4:2, 6:2, 8:2, 10;2, 12:2, 14:2, etc. (N:2 indicates N perfluorinated carbons attached to a non-fluorinated two-carbon unit -(CH2)2-. This is then purified by fractional distillation yielding a fraction containing the shorter chain lengths, i.e., C4-C10 mainly consisting of C6/C8, which has been used mainly for firefighting foams, and longer chain lengths >C8 used for fabric, textile, leather and paper treatments. Other structural variants on the telomer process have occasionally been used by individual manufacturers, including the use of a three carbon spacer, -(CH2)3-, instead of a two carbon unit.
Post the 2010-2015 PFOA Stewardship Program considerable efforts by the fluorochemical industry have managed to reduce the 8:2 fluorotelomer content of the precursor used for firefighting foams to less than 25ppb, as this can act as a precursor for PFOA through breakdown. Early products used to make the fluorosurfactants for formulating firefighting foams were actually a mixture of mainly C6/C8 perfluorinated chain lengths, i.e., 6:2 and 8:2. Modern fluorotelomer foams are now predominantly 6;2 and 4:2 and referred to in the industry as ‘’pure C6’’.
Unfortunately, but predictably, replacement of C6/C8 formulations with ’pure’ C6 fluorotelomers resulted in loss of foam performance which in turn required the use of higher fluorosurfactant concentrations, itself undesirable from an environmental point of view.
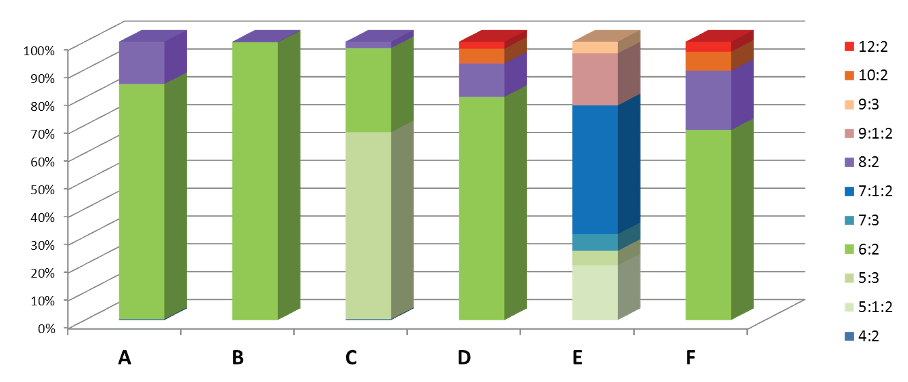
Compositions of six foams ~2005-2010. Data from Backe, Day & Field 2013
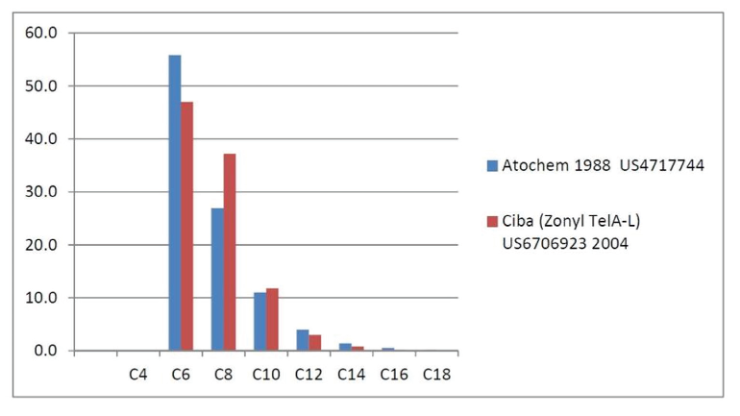
Fluorotelomer Intermediate Homologue Distributions
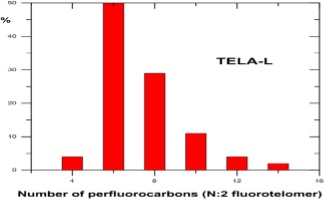
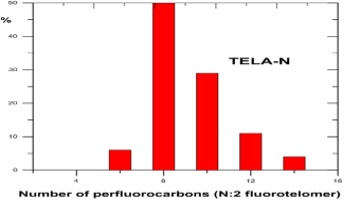
Source : DuPont
Perfluoro compounds are used in firefighting foam to lower surface tension enabling film-formation on many hydrocarbon fuels except those shorter than iso-octane such as hexane or pentane; to provide excellent heat and chemical resistance; to increase hydrocarbon repellency and thus to resist to solvent contamination or ‘f’uel pickup’’; to provide effective vapour suppression.
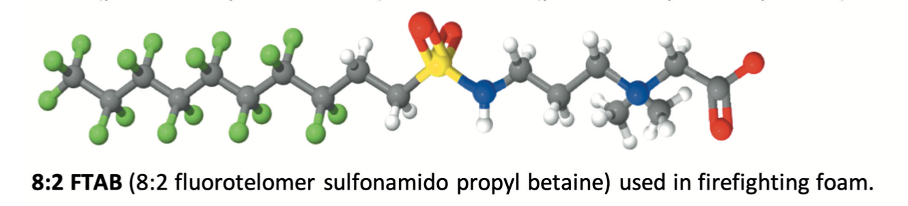
Manufacturers offer a range of perfluorochemicals, most of them being fluorosurfactants. These surfactants are a combination of a hydrophobic and oleophobic perfluorinated tail and a polar head group giving functionality, enabling dispersion or solubilisation of the products in the foam concentrate. One of the most popular and efficient products was probably the C8:2 perfluorinated betaine surfactant together with its C6:2 homologue.
The starting material is the perfluoroalkyl iodide, whereas the reactive end-product fluorotelomer iodide is used to manufacture a range of end products, e.g., fluorotelomer alcohols, thiols, sulfonic acids and sulfonamides.
Class B Fluorine-Free Foams (F3) for liquid hydrocarbons and polar solvents
The development of fluorine-free foams (F3) was started in the late 1990s by Ted Schaefer working for 3M Australia. By the early 2000s the first operational fluorine-free firefighting foam, called RF-3 and RF-6 for Rehealing foam 3% and 6% became available. Queensland Fire Service went fluorine-free as early as 2003. Over the next decade or so fluorine-free foam technology greatly improved to the point that today F3 products are available on the market achieving or even in some cases exceeding AFFF performance, whilst offering better value for money. Early developments included Solberg Scandinavian buying the RF patents from 3M as well as Ted Schaefer’s expertise in 2007, as well as the development of F3 by Thierry Bluteau in 2002, then working for Bio-Ex France. In the late 2000s, Gary McDowall (3F Ltd, UK) also developed F3 products. Later on, 3F Ltd offered new F3 solvent-free, i.e., glycol free, thus greatly reducing the BOD-COD problem by around 40-60%. Other major manufacturers followed suite and today F3 firefighting foams are widely available on the market, with many major organisations in civilian and military aviation, oil and gas and petrochemical industries, as well as large municipal fire departments transitioning from fluorine-containing AFFF to fluorine-free F3 foams.
The transition has taken nearly 10-15 years, mainly due to built-in conservatism in many fire departments, but also because of the costs involved which include modifying or cleaning existing equipment, as well as the proper and expensive disposal of existing legacy stocks of AFFF. Another driving force, especially in the US, has been the increasing financial and legal exposure of continuing to use products which give rise to persistent and widespread environmental contamination.
The environmentally sustainable destruction of legacy AFFF stocks, often involving huge volumes of concentrate running to millions of litres, requires destruction methods that are highly efficient (> 99.999% DRE), capable of handling solid and liquid charges, do not further contaminate the environment, and are financially feasible. Methods that are currently available will be discussed in a further article.
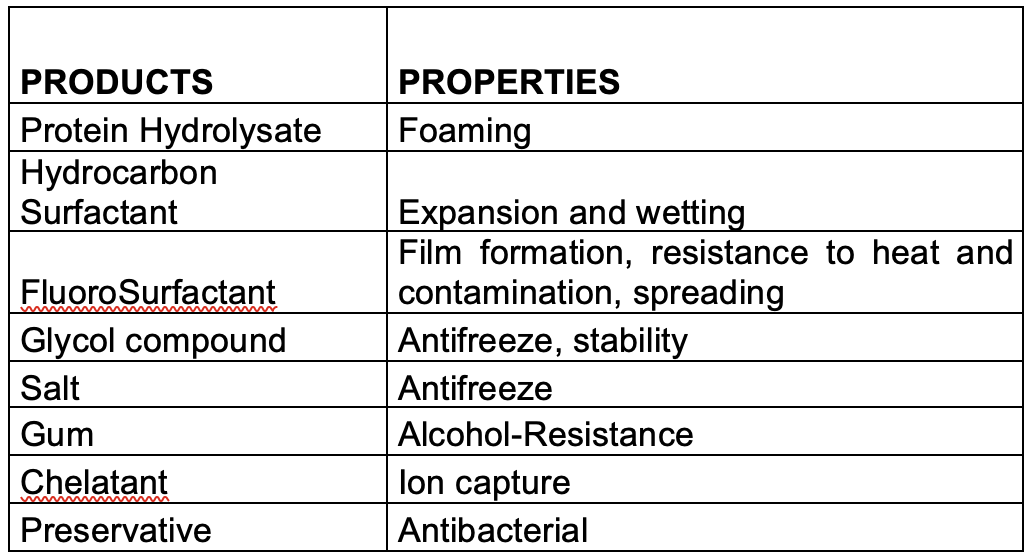
Apart from using fluorocompounds for their exceptional physicochemical properties, firefighting foam contains a range of other chemicals which are necessary to achieve the extinction.
The main components found in firefighting foam with or without fluorosurfactants or fluoropolymers include the following:
Foaming agents:
(a)Some fluorosurfactants like PFOS and PFHxS and their functionalised derivatives, or fluorotelomer compounds such as 1157 (perfluoroalkyl betaine) or 1183 (perfluoroalkyl aminoxide) have been used occasionally to boost foam volume in AFFF foams.
(b)r A large range of hydrocarbon surfactants are widely used by manufacturers in all types of synthetic foams: AFFF, AFFF-AR, High Expansion, Class A and F3.
Synthetic surfactants: are made from hydrocarbon chain precursors (e.g., CH3(CH2)n-produced by the petrochemical industry from mineral oil and/or animal and plant fatty acids, which are then functionalized with a polar head-group to obtain the desired surfactant property, for example, octyl sulfonate, CH3(CH2)7SO3-, or dodecyl sulfate, CH3(CH2)11SO4-.
(c) Protein polymer: obtained from the hydrolysis of slaughterhouse waste ‘‘horn and hoof’’, this old-fashioned and polluting process consists of heating the raw material in highly alkaline media. The keratin is degraded into small protein fragments, followed by neutralisation and stabilisation. The concentrated end-product can be contaminated with haemoglobin from residual blood giving it a very characteristic dark brown colour. Under operational conditions protein foams are characteristically dark brown in colour with a highly distinctive smell especially when applied to a fire.
Foam stabilisers: most of them are glycol ethers. The most used are butyl glycol, butyl carbitol and hexylene glycol, and more recently ethyl or butyl propylene glycols. We can find too lauryl alcohol.
Anti-freeze agents: monoethylene glycol, (CH2OH)2, and mono-propylene glycol, CH2(CH2OH)2, are widely used, but manufacturers also use sodium chloride, urea, etc, in some formulations.
The glycols and glycol ethers present in foam formulations are at relatively high concentrations – typically 10-20% – and are the major contributors to the BOD/COD value.
Other additives: in this category formulators use preservatives, anti-corrosion products, buffers to stabilise foam pH, and chelating agents for ions that would degrade foam performance, all at levels below 1%.
Natural polymers: carbohydrate xanthan gum is a very common natural polymer used to give alcohol-resistance to the foam. Applied to a burning fuel surface, the polymer precipitates and chars forming a barrier which resists and prevents contamination of the foam blanket by fuel – ‘’fuel pickup’’. Other polymers and gums are also used, such as celluloses, alginates, guar, locust bean, or carrageenan.
The tables below summarise the main properties of principal ingredients used in formulations.
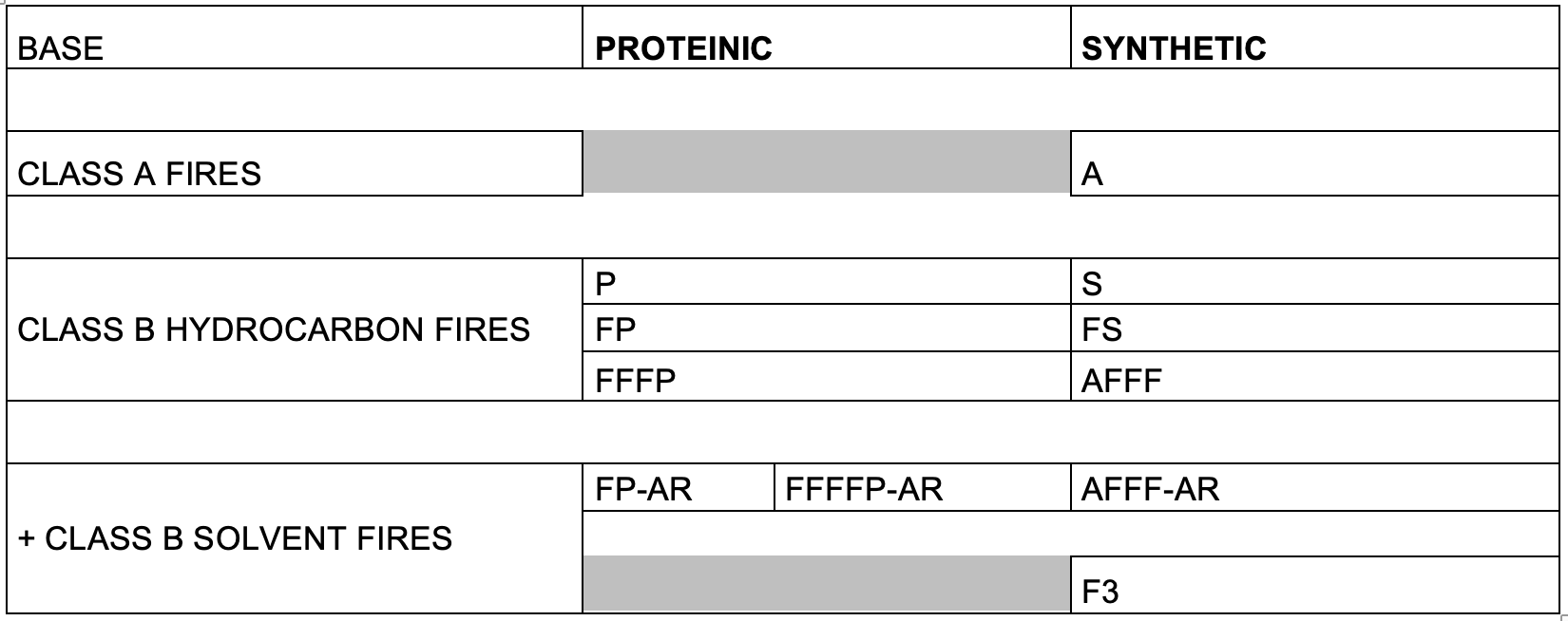
Currently, there are at least 12 different types or foam on the market, some of which have declined in the volume used over recent years.
Different users have different hazards associated with specific risks. In selecting the correct foam, it is important to do a suitable and sufficient assessment of these specific risks, ensuring that the foam chosen is ‘fit-for-purpose’, and then go through the following steps during procurement and operational use:
(a) list the equipment: whether this is fixed or mobile, i.e., tank farm, monitors or fire appliances;
(b) check the correct induction rate, e.g., 1%, 3% or 6%, for use;
(c) ensure that the application rate is suitable;
(d) determine the length of time that the foam should be applied, and the foam blanket stability and when re-application is necessary;
(e) determine the availability of possible support from external sources, i.e,. reinforcement;
(f) be aware of the manufacturer’s warranty and specified operating conditions for the foam;
(g) consider local environmental regulations – both current and any likely
3F is a responsible manufacturer and will be pleased to assist any of its customers in the assessment of risks and selection of an appropriate foam and associated equipment.
To be continued in Part 4.
References
Benskin J.P., De Silva A.O., Martin J.W. (2010) Isomer Profiling of Perfluorinated Substances as a Tool for Source Tracking: A Review of Early Findings and Future Applications. Rev. Environ. Contam. Toxicol.:111-160.
Buck, R.C., Franklin, J., Berger, U., Conder, J.M., Cousins, I.T., de Voogt, P., Jensen, A.A., Kannan, K., Mabury, S.A., and van Leeuwen, S.P.J. (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 7(4), 513-541.
D’Agostino, L.A, and Mabury, S.A. (2014) Identification of Novel Fluorinated Surfactants in Aqueous Film Forming Foams and Commercial Surfactant Concentrates. Environ. Sci. Technol. 48(1):121-9.
Moe, M.K., Huber, S., Svensen, J., Hagenaars, A,. Pabon, M., Trümper, M., Berger, U., Knapen, D., and Herzke, D. (2012) The structure of the fire fighting foam surfactant Forafac®1157 and its biological and photolytic transformation products. Chemosphere 89(7), 869-875.
Naile, J., Garrison, A.W., Avants, J.K., and Washington, J.W. Isomers/Enatiomers of Perfluorcarboxylic Acids: Method Development and Detection in Environmental Samples. Chemosphere 44, 1722-1728.
Sasaki, T., Egami, A., Yajima, T., Uekusa, H., and Sato, H. (2018) Unusual Molecular and Supramolecular Strcuturs of Chiral Low Molecular Weight Organogelators with Long Perfluoroalkyl Chains. Crystal Growth and Design 18(7) 4200-4205.
